The Design-Build-Test-Learn (DBTL) Cycle in Metabolic Engineering: A Framework for Accelerating Strain Development
This article provides a comprehensive overview of the Design-Build-Test-Learn (DBTL) cycle, a foundational and iterative framework in modern metabolic engineering.
The Design-Build-Test-Learn (DBTL) Cycle in Metabolic Engineering: A Framework for Accelerating Strain Development
Abstract
This article provides a comprehensive overview of the Design-Build-Test-Learn (DBTL) cycle, a foundational and iterative framework in modern metabolic engineering. Tailored for researchers, scientists, and drug development professionals, it explores the core principles of the DBTL cycle, detailing its application in optimizing microorganisms for the production of valuable compounds, from antibiotics to biotherapeutics. The content delves into methodological advancements, including the integration of automation and machine learning, addresses common challenges and optimization strategies to escape 'involution' cycles, and validates the approach through comparative case studies and performance analysis. By synthesizing foundational knowledge with current trends, this article serves as a guide for implementing efficient DBTL cycles to streamline bioprocess development and accelerate therapeutic discovery.
Foundations of the DBTL Cycle: The Core Engine of Modern Metabolic Engineering
The design-build-test-learn (DBTL) cycle is a foundational, iterative framework in metabolic engineering and synthetic biology used to develop and optimize microbial strains for the production of valuable compounds [1]. By systematically cycling through four defined phases—Design, Build, Test, and Learn—researchers can efficiently navigate complex biological systems to enhance product titers, yields, and productivity (TYR) [1]. This iterative process is central to modern biofoundries and is increasingly augmented by machine learning (ML) and automation, which help to overcome challenges such as combinatorial explosion of the design space and the costly nature of experimental trials [1] [2]. This guide details the technical execution of each phase within the context of metabolic engineering for a professional audience.
The Design Phase
The Design phase involves the rational selection of genetic targets and the planning of genetic constructs for the subsequent Build phase. The goal is to propose specific genetic modifications expected to improve microbial performance.
- Objective and Process: The objective is to select engineering targets, such as genes to be knocked out, overexpressed, or modulated. In classical metabolic engineering, this often involves sequential debottlenecking of rate-limiting steps. However, combinatorial pathway optimization, which targets multiple components simultaneously, reduces the chance of missing the global optimum pathway configuration [1]. Initial designs can be informed by prior knowledge, hypotheses, or computational models. In a knowledge-driven DBTL approach, upstream in vitro investigations in cell lysate systems can be used to assess enzyme expression levels and inform the initial design for the in vivo environment [3].
- Key Methodologies and Tools:
- Mechanistic Kinetic Modeling: Using ordinary differential equation (ODE) models to simulate pathway behavior and predict the effect of perturbations, such as changes in enzyme concentration, on metabolic flux [1].
- Machine Learning and AI: ML models can propose new designs by learning from data generated in previous DBTL cycles. More recently, large language models (LLMs) trained on protein sequences, such as ESM-2, are used to predict the fitness of protein variants, aiding in the design of high-quality mutant libraries for enzyme engineering [2].
- Library Design: For pathway optimization, designs are often based on a DNA library of components (e.g., promoters, ribosomal binding sites) that affect enzyme levels [1]. Tools like the UTR Designer can be used to modulate RBS sequences for fine-tuning gene expression [3].
The Build Phase
The Build phase is the physical implementation of the designed genetic constructs in the host organism. This phase is increasingly automated in biofoundries to ensure high throughput and reproducibility.
- Objective and Process: The objective is to rapidly and accurately assemble the designed genetic constructs and introduce them into the microbial host to create a library of strains. Automation is key to handling combinatorial libraries [2].
- Key Methodologies and Tools:
- Automated Molecular Cloning: Biofoundries, such as the Illinois Biological Foundry for Advanced Biomanufacturing (iBioFAB), use fully automated, modular workflows for cloning. This includes automated modules for mutagenesis PCR, DNA assembly, transformation, and colony picking [2].
- Genetic Toolkits: Common techniques include:
- Ribosome Binding Site (RBS) Engineering: A powerful method for fine-tuning the relative expression levels of genes within an operon. This can be achieved by modulating the Shine-Dalgarno sequence to alter the translation initiation rate without significantly affecting secondary structures [3].
- Site-Directed Mutagenesis (SDM): For protein engineering, a high-fidelity (HiFi) assembly-based mutagenesis method can be used to create variant libraries without the need for intermediate sequence verification, enabling a continuous workflow [2].
- Genome Engineering: Using CRISPR/Cas systems or other methods to make genomic modifications, such as knocking out regulatory genes (e.g., tyrR) or mutating feedback inhibition (e.g., in tyrA) to increase precursor availability [3].
The Test Phase
The Test phase involves cultivating the newly built strains and characterizing their performance through analytical methods to collect high-quality data.
- Objective and Process: The objective is to measure the fitness or performance of the engineered strains, typically by quantifying the production of the target compound (titer), biomass yield, and growth rate. This data is essential for the subsequent Learn phase.
- Key Methodologies and Tools:
- Cultivation Systems: Strains are cultivated in controlled bioreactors, from small-scale microtiter plates to 1 L batch reactors, to monitor biomass growth and substrate consumption [1] [3].
- Analytical Chemistry: Techniques like High-Performance Liquid Chromatography (HPLC) are used to quantify extracellular metabolites, precursors, and products (e.g., L-tyrosine, L-DOPA, dopamine) [3].
- Advanced Metabolomics:
- Mass Spectrometry Imaging (MSI): Methods like "RespectM" enable single-cell level metabolomics, detecting metabolites from hundreds of cells per hour. This reveals metabolic heterogeneity within a cell population, generating large datasets that can power deep learning models [4].
- Cell-Free Protein Synthesis (CFPS): Crude cell lysate systems can be used to test pathway enzyme expression and function in vitro, bypassing whole-cell constraints [3].
Table 1: Key Performance Metrics in the Test Phase
| Metric | Description | Example Measurement |
|---|---|---|
| Titer | Concentration of the target product in the fermentation broth | 69.03 ± 1.2 mg/L of dopamine [3] |
| Yield | Amount of product per unit of biomass | 34.34 ± 0.59 mg/g˅biomass of dopamine [3] |
| Productivity | Rate of product formation | Often reported as mg/L/h |
| Enzyme Activity | Catalytic efficiency of engineered enzymes | 26-fold improvement in phytase activity at neutral pH [2] |
| Metabolic Heterogeneity | Variation in metabolite levels across a cell population | 4,321 single-cell metabolomics data points [4] |
The Learn Phase
The Learn phase is where data from the Test phase is analyzed to extract insights, update models, and generate new hypotheses to inform the design of the next DBTL cycle.
- Objective and Process: The objective is to learn important characteristics of the engineered pathway or enzyme from the experimental data. The complexity of biological systems often means that the outcomes of genetic perturbations are non-intuitive, making this a critical phase [1].
- Key Methodologies and Tools:
- Machine Learning: Supervised ML models are trained on the experimental data to predict strain performance based on genetic design.
- Model Training: In the low-data regime typical of early DBTL cycles, gradient boosting and random forest models have been shown to be robust to training set biases and experimental noise [1].
- Heterogeneity-Powered Learning (HPL): Single-cell metabolomics data, representing metabolic heterogeneity, can be used to train deep neural networks (DNNs). These HPL-based models can then suggest minimal genetic operations to achieve a desired metabolic output, such as high triglyceride production [4].
- Recommendation Algorithms: Once a model is trained, algorithms are used to recommend the most promising designs for the next DBTL cycle. These algorithms balance exploration (testing new regions of the design space) and exploitation (focusing on areas with predicted high performance) [1].
- Machine Learning: Supervised ML models are trained on the experimental data to predict strain performance based on genetic design.
Table 2: Machine Learning Models Used in the Learn Phase
| Model/Algorithm | Application in DBTL Cycles | Key Strength |
|---|---|---|
| Gradient Boosting | Predicting strain performance from genetic design data [1] | High predictive performance with small datasets |
| Random Forest | Predicting strain performance from genetic design data [1] | Robust to noise and bias in training data |
| Deep Neural Network (DNN) | Learning from single-cell metabolomics data (HPL) [4] | Can model complex, non-linear relationships in large datasets |
| Epistasis Model (EVmutation) | Guiding the design of protein variant libraries [2] | Uses evolutionary sequences to predict mutation effects |
| Protein LLM (ESM-2) | Designing initial protein variant libraries [2] | Predicts amino acid likelihoods from sequence context |
DBTL Workflow and Cycle Strategies
The following diagram illustrates the integrated, iterative workflow of a DBTL cycle, incorporating automated and AI-powered elements.
Strategy for Efficient Cycling: A key operational question is how to allocate resources across multiple DBTL cycles. Simulation studies using kinetic models suggest that when the total number of strains to be built is limited, it is more effective to start with a large initial DBTL cycle rather than distributing the same number of strains evenly across every cycle [1]. This initial large dataset provides a more robust foundation for the machine learning models in the Learn phase, leading to better recommendations in subsequent cycles.
Essential Research Reagent Solutions
The following table details key reagents, tools, and resources essential for executing a DBTL cycle in metabolic engineering.
Table 3: Key Research Reagent Solutions for DBTL Cycles
| Item | Function/Description | Example Use |
|---|---|---|
| RBS Library | A predefined set of ribosomal binding site sequences used to fine-tune the translation initiation rate of genes. | Fine-tuning expression of hpaBC and ddc genes in a dopamine pathway [3]. |
| Promoter Library | A collection of promoter sequences of varying strengths to control transcription levels of pathway genes. | Combinatorial optimization of enzyme concentrations in a synthetic pathway [1]. |
| pET / pJNTN Plasmid Systems | Common plasmid vectors used for heterologous gene expression in E. coli. | Serving as storage vectors for genes or for constructing plasmid libraries for pathway expression [3]. |
| Cell-Free Protein Synthesis (CFPS) System | A crude cell lysate system used for in vitro transcription and translation, bypassing whole-cell constraints. | Testing relative enzyme expression levels and pathway function in vitro before DBTL cycling [3]. |
| Mass Spectrometry Imaging (MSI) | An analytical technique for detecting and visualizing the spatial distribution of metabolites. | Acquiring single-cell level metabolomics data (e.g., using RespectM) to study metabolic heterogeneity [4]. |
| Automated Biofoundry (e.g., iBioFAB) | An integrated robotic platform for automating laboratory processes in synthetic biology. | Executing end-to-end protein engineering workflows, from library construction to functional assays [2]. |
| Machine Learning Models (e.g., ESM-2, EVmutation) | Computational models used to predict the effect of genetic changes on protein function or pathway performance. | Designing high-quality initial mutant libraries for enzyme engineering campaigns [2]. |
The DBTL cycle is a powerful, iterative framework that structures the scientific and engineering process in metabolic engineering. Its effectiveness is greatly enhanced by the integration of automation, high-throughput analytics, and artificial intelligence. As these technologies continue to advance, they will further accelerate the DBTL cycle, reducing the time and cost required to develop robust microbial cell factories for the production of pharmaceuticals, biofuels, and sustainable chemicals.
The Design-Build-Test-Learn (DBTL) cycle represents a systematic framework for optimizing microbial cell factories in metabolic engineering. This iterative process enables researchers to progressively enhance strain performance through consecutive rounds of design intervention, genetic construction, phenotypic testing, and data analysis. Recent advances demonstrate how the DBTL cycle, particularly when augmented with upstream knowledge and mechanistic insights, accelerates the development of high-yielding strains for bio-based production. This technical guide examines the core principles and implementation strategies of the DBTL framework, highlighting its spiral nature where each iteration generates valuable knowledge that informs subsequent cycles, ultimately driving continuous improvement toward optimal strain performance.
The DBTL cycle has emerged as a cornerstone methodology in modern metabolic engineering and synthetic biology, providing a structured approach to strain development. This engineering paradigm integrates tools from synthetic biology, enzyme engineering, omics technologies, and evolutionary engineering to optimize metabolic pathways in microbial hosts [5]. The cyclic nature of this process distinguishes it from traditional linear approaches, creating a feedback loop where learning from each test phase directly informs the subsequent design phase. This iterative refinement enables researchers to navigate the complexity of biological systems methodically, addressing multiple engineering targets while accumulating mechanistic understanding of pathway regulation and host physiology.
In industrial biotechnology, the DBTL framework has revolutionized the development of microbial cell factories as sustainable alternatives to traditional petrochemical processes [5]. The cycle begins with rational design based on available knowledge, proceeds to physical construction of genetic variants, advances to rigorous phenotypic testing, and culminates in data analysis that extracts meaningful insights for the next iteration. The power of this approach lies in its flexibility—it can be applied across different microbial platforms, from well-established workhorses like Corynebacterium glutamicum and Escherichia coli to non-conventional organisms, with each spiral of the cycle propelling the strain closer to its performance targets.
Deconstructing the DBTL Cycle: Phase-by-Phase Analysis
Design Phase: Rational Planning of Strain Engineering
The Design phase establishes the foundational blueprint for strain modification, combining computational tools, prior knowledge, and strategic planning. In metabolic engineering projects, this typically involves identifying target pathways, selecting appropriate enzymes, choosing regulatory elements, and predicting potential metabolic bottlenecks. Modern design strategies increasingly incorporate in silico modeling and bioinformatics tools to prioritize engineering targets, moving beyond random selection toward hypothesis-driven approaches [3]. The design phase may also include enzyme engineering strategies to alter substrate specificity or improve catalytic efficiency, and genome-scale modeling to predict system-wide consequences of pathway manipulations.
A significant advancement in this phase is the "knowledge-driven DBTL" approach, which incorporates upstream in vitro investigations before committing to genetic modifications in the production host [3]. For instance, researchers developing dopamine-producing E. coli strains first conducted cell lysate studies to assess enzyme expression levels and pathway functionality under controlled conditions. This pre-validation enables more informed selection of engineering targets for the subsequent in vivo implementation, potentially reducing the number of DBTL iterations required to achieve optimal performance. The design phase thus transforms from a purely computational exercise to an experimentally informed strategy that de-risks the subsequent build and test phases.
Build Phase: Genetic Construction of Engineered Strains
The Build phase translates design specifications into physical biological entities through genetic engineering. This stage encompasses the assembly of DNA constructs, pathway integration into host chromosomes, and development of variant libraries for testing. Advanced modular cloning techniques and automated DNA assembly platforms have dramatically accelerated this phase, enabling high-throughput construction of genetic variants [3]. For metabolic pathways, this often involves combining multiple enzyme-coding genes with appropriate regulatory elements into coordinated expression systems.
A key build strategy featured in recent implementations is ribosome binding site (RBS) engineering for fine-tuning gene expression in synthetic pathways [3]. By modulating the Shine-Dalgarno sequence without altering the coding sequence or creating secondary structures, researchers can precisely control translation initiation rates for optimal metabolic flux. In the dopamine production case study, researchers created RBS libraries to systematically vary the expression levels of the hpaBC and ddc genes, enabling identification of optimal expression ratios for maximal dopamine yield [3]. The build phase increasingly leverages automation and standardized genetic parts to enhance reproducibility and scalability across multiple DBTL iterations.
Test Phase: Phenotypic Characterization of Engineered Strains
The Test phase involves rigorous experimental characterization of built strains to evaluate performance against design specifications. This encompasses cultivation experiments under controlled conditions, analytical chemistry techniques to quantify metabolites, and omics analyses to assess system-wide responses. For metabolic engineering projects, the test phase typically measures key performance indicators such as product titer, yield, productivity, and cellular fitness [3]. Advanced cultivation platforms enable parallel testing of multiple strain variants, generating robust datasets for the subsequent learning phase.
In the dopamine production case study, researchers employed minimal medium cultivations with precise monitoring of biomass and dopamine accumulation over time [3]. The test phase quantified both volumetric production (69.03 ± 1.2 mg/L) and specific production (34.34 ± 0.59 mg/gbiomass), representing a 2.6-fold and 6.6-fold improvement over previous reports, respectively. Similarly, in the C. glutamicum C5 chemical production platform, the test phase evaluated the performance of engineered strains in converting L-lysine to higher-value chemicals [5]. Comprehensive testing generates the essential data required for meaningful analysis in the learning phase, creating a direct link between genetic modifications and phenotypic outcomes.
Learn Phase: Data Analysis and Insight Generation
The Learn phase represents the critical knowledge extraction component of the cycle, where experimental data transforms into actionable insights. This stage employs statistical analysis, machine learning algorithms, and mechanistic modeling to identify relationships between genetic modifications and phenotypic outcomes [3]. The learning phase answers fundamental questions about which engineering strategies succeeded, which failed, and why—thereby generating hypotheses for the next design iteration. For researchers, this phase involves comparing experimental results with design predictions, identifying performance bottlenecks, and proposing new modification targets.
In the knowledge-driven DBTL approach, the learning phase extends beyond correlation to establish mechanistic causality [3]. For instance, dopamine production studies revealed how GC content in the Shine-Dalgarno sequence directly influences RBS strength and consequently pathway performance. The iGEM Engineering Committee emphasizes that in this phase, teams should "link your experimental data back to your design and complete the first iteration of the DBTL cycle," using the data to "create informed decisions as to what needs to be changed in your design" [6]. Effective learning requires both quantitative analysis of performance metrics and qualitative understanding of biological mechanisms that explain the observed phenotypes.
Quantitative Analysis of DBTL Implementation
Table 1: Performance Metrics from DBTL-Optimized Dopamine Production in E. coli [3]
| Strain Generation | Dopamine Titer (mg/L) | Specific Dopamine Production (mg/gbiomass) | Fold Improvement Over Baseline |
|---|---|---|---|
| Baseline (Literature) | 27.0 | 5.17 | 1.0 |
| DBTL-Optimized | 69.03 ± 1.2 | 34.34 ± 0.59 | 2.6 (titer), 6.6 (specific) |
Table 2: Clay Prototype Comfort Ratings for Pipette Grip Design [7]
| Mold Iteration | Thin Section (mm) | Mid Section (mm) | Thick Section (mm) | Comfort Rating (out of 10) |
|---|---|---|---|---|
| 1 | 7.24 | 11.0 | 10.55 | 8 |
| 2 | 6.35 | 19.0 | 14.34 | 8 |
| 3 | 10.78 | (missed) | 37.0 | 2 |
| 4 | 10 | 26 | 13 | 4.5 |
| 5 | without clay | without clay | without clay | 5 |
| 6 | 7.54 | 23.05 | 14.15 | 6 |
| 7 | 5.65 | 13.38 | 19.68 | 8.2 |
| 8 | 10.47 | 10.47 | 11.11 | 10 |
Experimental Protocols for DBTL Implementation
Knowledge-Driven DBTL with Upstream In Vitro Testing
The knowledge-driven DBTL cycle incorporates upstream in vitro investigation before proceeding to in vivo strain engineering [3]. This protocol begins with preparation of crude cell lysate systems from potential production hosts. The reaction buffer is prepared with 50 mM phosphate buffer (pH 7) supplemented with 0.2 mM FeCl₂, 50 µM vitamin B6, and pathway-specific substrates (1 mM L-tyrosine or 5 mM L-DOPA for dopamine production) [3]. Heterologous genes are cloned into appropriate expression vectors (e.g., pJNTN system) and expressed in the lysate system. Pathway functionality is assessed by measuring substrate conversion and product formation rates, enabling preliminary optimization of enzyme ratios and identification of potential bottlenecks before genetic modification of the production host.
Following in vitro validation, the protocol proceeds to high-throughput RBS engineering for in vivo implementation. Genetic constructs are designed with modular RBS sequences varying in Shine-Dalgarno composition while maintaining constant coding sequences. Library construction employs automated DNA assembly techniques, with transformation into appropriate production hosts (e.g., E. coli FUS4.T2 for dopamine production) [3]. Strain cultivation utilizes minimal medium containing 20 g/L glucose, 10% 2xTY medium, phosphate buffer, MOPS, vitamin B6, phenylalanine, and essential trace elements. Cultivation proceeds with appropriate antibiotics and inducers (e.g., 1 mM IPTG), followed by analytical measurement of target metabolites to identify top-performing variants for the next DBTL iteration.
Iterative Prototyping for Hardware-Design Integration
The DBTL cycle also applies to hardware development complementing biological engineering, as demonstrated by the UBC iGEM team's pipette add-on project [7]. The protocol begins with preliminary CAD modeling based on user needs assessment (Design phase). The Build phase employs rapid prototyping with accessible materials like air-dry clay to create physical models for initial user testing. The Test phase involves structured user interviews with quantitative comfort ratings recorded for different design iterations (see Table 2). During interviews, users physically interact with prototypes and provide comfort feedback, enabling dimensional optimization.
The Learn phase employs decision matrices to translate qualitative user feedback into quantitative design parameters [7]. For the pipette project, this revealed that "reducing the need for extensive gripping" was the highest priority (60% weight), followed by maintaining low weight (28% weight), using soft materials (8% weight), and reducing knob pressure (4% weight) [7]. This learning directly informed the next design iteration, with prototype modifications focusing on these weighted parameters. The process demonstrates how DBTL cycles effectively integrate user-centered design into biological engineering projects.
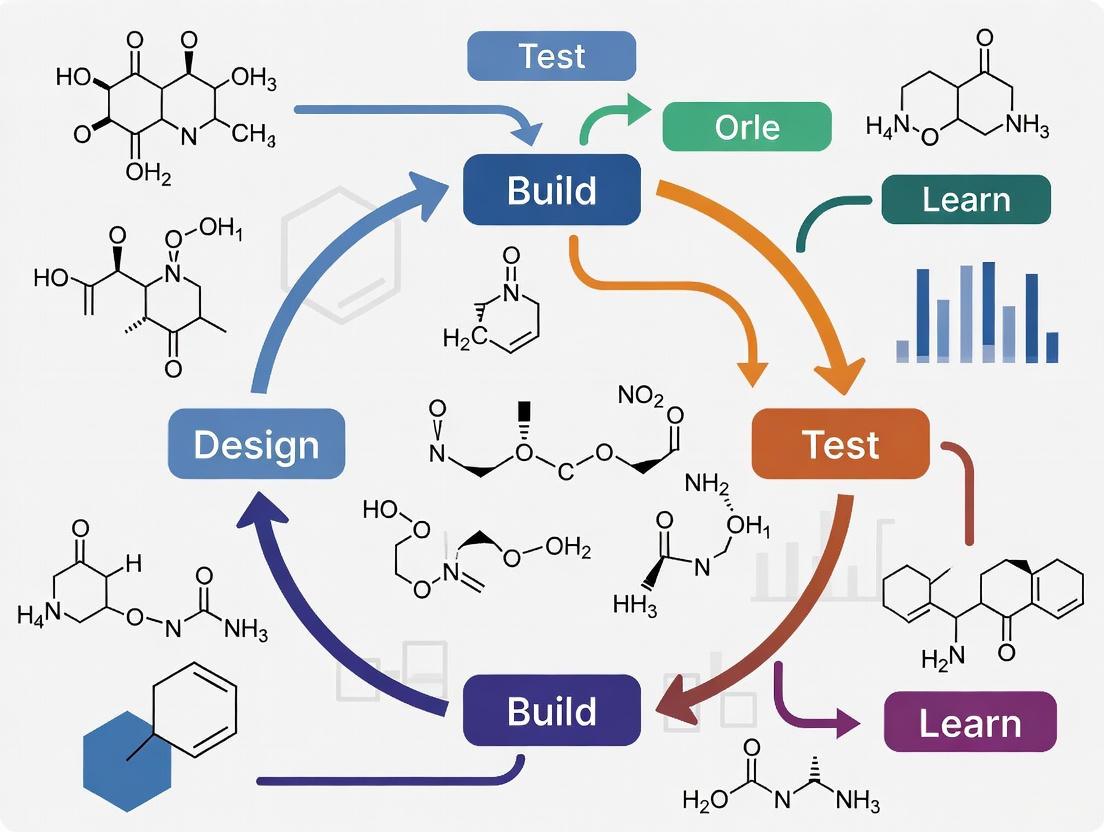
Visualizing DBTL Workflows and Relationships
Diagram 1: The Core DBTL Cycle in Metabolic Engineering
Diagram 2: Knowledge-Driven DBTL with Upstream In Vitro Testing
Table 3: Key Research Reagent Solutions for DBTL Implementation
| Reagent/Resource | Function in DBTL Cycle | Application Example |
|---|---|---|
| Crude Cell Lysate Systems | Enables in vitro pathway testing before in vivo implementation | Testing enzyme expression levels and pathway functionality [3] |
| RBS Library Kits | Facilitates fine-tuning of gene expression in metabolic pathways | Modulating translation initiation rates for optimal metabolic flux [3] |
| Minimal Medium Formulations | Provides controlled cultivation conditions for phenotype testing | Assessing strain performance under defined nutritional conditions [3] |
| Analytical Standards | Enables accurate quantification of metabolites and products | Measuring dopamine production titers via HPLC or LC-MS [3] |
| CAD Software | Supports hardware design for experimental automation | Creating 3D models of custom lab equipment [7] |
| Data Analysis Platforms | Facilitates learning phase through statistical analysis | Using R, MATLAB, or Python for data processing and visualization [6] |
The iterative nature of the DBTL cycle creates a spiral of continuous improvement in metabolic engineering, where each iteration builds upon knowledge gained from previous cycles. This structured approach transforms strain development from a trial-and-error process to a systematic engineering discipline, efficiently navigating the complexity of biological systems toward optimal performance. The integration of upstream knowledge generation, automated workflows, and multi-omic analyses further enhances the efficiency of each DBTL iteration, accelerating the development of microbial cell factories for sustainable bioproduction. As DBTL methodologies continue to evolve with advances in synthetic biology and automation, they will undoubtedly remain central to the optimization of strain performance for industrial and pharmaceutical applications.
Overcoming Combinatorial Explosions in Pathway Optimization
Metabolic engineering aims to reprogram microbial metabolism to produce valuable compounds, from pharmaceuticals to sustainable fuels [8]. A fundamental strategy involves introducing heterologous pathways or optimizing native ones. However, engineering these pathways often reveals significant imbalances in metabolic flux, leading to the accumulation of toxic intermediates, side products, and suboptimal yields [8]. Classical "de-bottlenecking" approaches address these limitations sequentially. While sometimes successful, this method often fails to find a globally optimal solution for the pathway because it neglects the complex, holistic interactions between multiple pathway components and the host's native metabolism [8] [1].
Combinatorial pathway optimization has emerged as a powerful alternative, enabled by dramatic reductions in the cost of DNA synthesis and advances in DNA assembly and genome editing [8]. This approach involves the simultaneous diversification of multiple pathway parameters—such as enzyme homologs, gene copy number, and regulatory elements—to create vast libraries of genetic variants [8]. The major constraint of this method is combinatorial explosion, where the number of potential permutations increases exponentially with the number of components being optimized [8] [1]. For example, diversifying just 10 pathway elements with 5 variants each generates 9,765,625 (5^10) unique combinations, making exhaustive screening experimentally infeasible [1].
The Design-Build-Test-Learn (DBTL) cycle provides a structured framework to navigate this vast design space efficiently. By iteratively applying this cycle, researchers can gradually steer the optimization process toward high-performing strains with manageable experimental effort [1] [3] [9]. This guide details the core objectives and methodologies for overcoming combinatorial explosions within the DBTL paradigm.
The DBTL Cycle: A Framework for Efficient Optimization
The DBTL cycle is an iterative engineering process that transforms the daunting task of combinatorial optimization into a manageable, data-driven workflow. Its power lies in using information from each cycle to intelligently guide the design of the next, progressively focusing on a more promising and smaller region of the design space.
Table: The Four Phases of the DBTL Cycle and Their Role in Combating Combinatorial Explosion
| DBTL Phase | Core Objective | Key Activities | How It Addresses Combinatorial Explosion |
|---|---|---|---|
| Design | Plan a library of genetic variants based on prior knowledge or data. | Selection of enzyme homologs, promoters, RBS sequences, and gene order; Use of statistical design (DoE) to reduce library size. | Reduces the initial search space from millions to a tractable number (e.g., 10s-100s) of representative constructs. |
| Build | Physically construct the designed genetic variants. | Automated DNA assembly, molecular cloning, and genome engineering. | Enables high-throughput, reliable construction of variant libraries, often leveraging robotics. |
| Test | Characterize the performance of the built variants. | Cultivation in microplates, automated metabolite extraction, analytics (e.g., LC-MS), and product quantification. | Generates high-quality data linking genotype to phenotype (e.g., titer, yield, rate) for the screened library. |
| Learn | Analyze data to extract insights and generate new hypotheses. | Statistical analysis, machine learning (ML) model training, and identification of limiting factors or optimal patterns. | Creates a predictive model of pathway behavior, which is used to design a more efficient library in the next cycle. |
The following diagram illustrates the logical workflow and information flow of an iterative DBTL cycle, highlighting how learning from one cycle directly informs the design of the next.
Core Strategies for Library Diversification
A primary lever for controlling combinatorial explosion is the strategic choice of which pathway elements to diversify. The goal is to maximize the potential for improvement while minimizing the number of variables.
Variation of Coding Sequences (CDS)
This strategy involves swapping the enzymes that catalyze each reaction. It is crucial when enzyme properties like catalytic efficiency, substrate specificity, or inhibitor sensitivity are unknown or suspected to be suboptimal.
- Methodology: Identify multiple structural or functional gene homologs from different organisms for each enzymatic step in the pathway. These homologs can be sourced from public databases or metagenomic libraries [8]. For instance, to engineer xylose utilization in Saccharomyces cerevisiae, researchers screened a library of xylose isomerase homologs from various bacteria to identify the most functional variant in yeast [8].
- Experimental Protocol:
- In silico Identification: Use tools like BLAST or enzyme-specific databases (e.g., BRENDA) to identify potential homologs.
- Gene Synthesis: Commercially synthesize the selected coding sequences with codon optimization for the host chassis.
- Standardized Assembly: Clone each homolog into a standardized expression vector (e.g., with a fixed promoter and RBS) using high-throughput DNA assembly methods like Golden Gate or Gibson Assembly.
- Screening: Transform the library into the production host and screen for the desired phenotype (e.g., product titer, growth rate).
Engineering of Expression Levels
Fine-tuning the expression level of each pathway gene is often the most effective way to balance metabolic flux and prevent the accumulation of intermediates.
- Methodology: Key tunable elements include:
- Promoter Strength: Replacing the native promoter with a library of constitutive or inducible promoters of varying strengths [8] [9].
- Ribosome Binding Site (RBS) Engineering: Designing a library of RBS sequences with varying translation initiation rates (TIR) to control translational efficiency [8] [3]. Tools like the UTR Designer can assist in this process [3].
- Gene Dosage: Using plasmids with different origins of replication (copy numbers) or integrating varying gene copies into the genome [8] [9].
- Experimental Protocol (RBS Library Example):
- Library Design: Define a set of Shine-Dalgarno (SD) sequences with varying calculated strengths, ensuring minimal alteration to mRNA secondary structure [3].
- PCR-based Construction: Use overlap extension PCR or specialized cloning techniques (e.g., ligase cycling reaction) to generate a library of constructs where the target gene is preceded by different RBS variants.
- Characterization: Measure the resulting protein expression levels for a subset of variants via SDS-PAGE or fluorescence assays to validate the library's functional diversity.
Combined and Integrated Approaches
The most powerful optimization campaigns often simultaneously target multiple layers of regulation. For example, a single pathway can be optimized by combining the best-performing enzyme homologs with optimally tuned expression levels for each [8]. A notable example is the combinatorial refactoring of a 16-gene nitrogen fixation pathway, which involved the simultaneous optimization of promoters, RBSs, and gene order, leading to a significant improvement in function [8].
Key Methodologies for Managing Experimental Effort
Statistical Design of Experiments (DoE)
Instead of testing all possible combinations, DoE selects a representative subset of the full factorial library. This allows for the efficient exploration of the design space and the statistical identification of the main effects and interactions of each diversified component.
- Application: In one study optimizing a 4-gene flavonoid pathway, a combinatorial design of 2592 possible configurations was reduced to just 16 representative constructs using orthogonal arrays and a Latin square design—a compression ratio of 162:1. Screening this small library was sufficient to identify copy number and specific promoter strengths as the most critical factors influencing production [9].
Machine Learning (ML)-Guided Recommendation
Machine learning has become a cornerstone of the "Learn" phase, enabling semi-automated strain recommendation.
- Workflow: In the first DBTL cycle, an initial library of strains is built and tested to generate a dataset. An ML model (e.g., Random Forest, Gradient Boosting) is trained on this data to learn the complex relationships between genetic design features (e.g., promoter strength, RBS sequence) and phenotypic outcomes (e.g., titer) [1]. This model then predicts the performance of all possible, untested designs and recommends a shortlist of the most promising candidates for the next "Build" phase.
- Performance: Simulation studies show that ML models like gradient boosting and random forest are particularly effective in the low-data regime typical of early DBTL cycles and are robust to experimental noise [1]. An Automated Recommendation Tool (ART) that uses an ensemble of models has been successfully applied to optimize the production of compounds like dodecanol and tryptophan [1].
Knowledge-Driven and Hybrid Approaches
Incorporating prior mechanistic knowledge can dramatically improve the efficiency of the initial DBTL cycle.
- In Vitro Prototyping: Before moving to in vivo strain construction, pathway bottlenecks can be identified using cell-free transcription-translation systems (TXTL) or crude cell lysate systems [3]. For dopamine production in E. coli, researchers first used a cell lysate system to test different relative expression levels of the pathway enzymes. The insights gained directly informed the design of the in vivo RBS library, leading to a 2.6 to 6.6-fold improvement over the state-of-the-art in just one DBTL cycle [3].
- Kinetic Modeling: Mechanistic kinetic models of the pathway embedded in cell physiology can be used to simulate DBTL cycles in silico. This provides a framework for benchmarking ML algorithms and optimizing the DBTL strategy itself (e.g., determining the ideal number of strains to build per cycle) before committing to costly wet-lab experiments [1].
Table: Comparison of Strategies for Reducing Experimental Effort
| Strategy | Mechanism | Best-Suited Context | Advantages | Limitations |
|---|---|---|---|---|
| Design of Experiments (DoE) | Uses statistical principles to select a representative subset of all combinations. | Early DBTL cycles with many factors to explore; when factor interactions are unknown. | Efficiently identifies major influential factors with minimal experiments. | Limited ability to model highly non-linear, complex interactions compared to ML. |
| Machine Learning (ML) | Learns a non-linear model from data to predict high-performing designs. | Later DBTL cycles after initial data is available; complex pathways with interacting elements. | Can find non-intuitive optimal combinations; improves with each cycle. | Requires initial dataset; predictive performance can be poor with very small or biased data. |
| Knowledge-Driven Design | Uses upstream experiments (e.g., in vitro tests) or prior knowledge to constrain initial design. | Pathways with known toxic intermediates or well-characterized enzymes. | Reduces initial blind exploration; provides mechanistic insights. | Requires established upstream protocols; may introduce bias if knowledge is incomplete. |
The Scientist's Toolkit: Essential Research Reagents and Solutions
Table: Key Research Reagents for Combinatorial Pathway Optimization
| Reagent / Material | Function in Pathway Optimization |
|---|---|
| Commercial DNA Synthesis | Provides the raw genetic material for constructing variant libraries of coding sequences, promoters, and RBSs [8]. |
| Standardized Plasmid Vectors | Act as modular scaffolds for the assembly of pathway variants. Vectors with different origins of replication (e.g., ColE1, p15a, pSC101) allow for control of gene dosage [9]. |
| High-Throughput DNA Assembly Kits (e.g., Gibson Assembly, Golden Gate, LCR) | Enable the rapid, parallel, and often automated assembly of multiple DNA parts into functional constructs [8] [9]. |
| Cell-Free Transcription-Translation (TXTL) Systems | Used for in vitro prototyping of pathways to rapidly identify flux bottlenecks and inform in vivo library design without cellular constraints [3]. |
| Ribosome Binding Site (RBS) Library Kits | Pre-designed collections of RBS sequences with characterized strengths, used for fine-tuning translational efficiency of pathway genes [3]. |
| Analytical Standards (e.g., target product, pathway intermediates) | Essential for calibrating analytical equipment (e.g., LC-MS) and quantitatively measuring the performance of engineered strains during the Test phase [9]. |
Combinatorial explosion is not an insurmountable barrier but a fundamental characteristic of biological complexity that can be managed through a disciplined DBTL framework. The convergence of robust library diversification strategies, high-throughput automation, and sophisticated computational learning methods has transformed pathway optimization from a sequential, trial-and-error process into a rapid, iterative, and predictive engineering science. By strategically applying statistical design, machine learning, and mechanistic insights, researchers can systematically navigate the vast combinatorial search space to develop high-performing microbial cell factories with unprecedented efficiency.
The field of metabolic engineering has undergone a radical transformation, evolving from a purely descriptive science into a sophisticated design discipline. This evolution is characterized by the adoption of the Design-Build-Test-Learn (DBTL) cycle, a framework that has revolutionized both classic antibiotic discovery and contemporary bioproduction efforts. Where traditional antibiotic discovery in organisms like Streptomycetes often relied on observational methods and trial-and-error approaches, modern bioengineering leverages automated, iterative DBTL cycles to precisely optimize microbial strains for producing valuable compounds, from biofuels to pharmaceuticals [10] [11]. This shift has been enabled by technological advancements in genetic editing, automation, and data science, allowing researchers to systematically convert cellular factories into efficient producers of target molecules.
The DBTL cycle provides a structured framework for metabolic engineering experiments. In the Design phase, biological systems are conceptualized and modeled. The Build phase implements these designs in biological systems through genetic construction. The Test phase characterizes the performance of built strains, and the Learn phase analyzes data to inform the next design iteration [12]. This cyclic process has become the cornerstone of modern synthetic biology, enabling continuous improvement of microbial strains through successive iterations [9].
The DBTL Cycle: Core Components and Workflow
The DBTL cycle represents a systematic framework for metabolic engineering that has largely replaced the traditional, linear approaches to strain development. Each phase contributes uniquely to the iterative optimization process:
Design: This initial phase employs computational tools to select pathways and enzymes, design DNA parts, and create combinatorial libraries. Tools like RetroPath and Selenzyme facilitate automated enzyme selection, while PartsGenie designs reusable DNA components with optimized ribosome-binding sites and coding regions. Designs are statistically reduced using design of experiments (DoE) to create tractable libraries for laboratory construction [9].
Build: Implementation begins with commercial DNA synthesis, followed by automated pathway assembly using techniques like ligase cycling reaction (LCR) on robotics platforms. After transformation into microbial hosts, quality control is performed via automated purification, restriction digest, and sequence verification. This phase benefits from standardization through repositories like the Inventory of Composable Elements (ICE) [10] [9].
Test: Constructs are introduced into production chassis and evaluated using automated cultivation protocols. Target products and intermediates are detected through quantitative screening methods, typically ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS). Data extraction and processing are automated through custom computational scripts [9].
Learn: This crucial phase identifies relationships between design factors and production outcomes using statistical methods and machine learning. The insights generated inform the next Design phase, creating a continuous improvement loop. Modern implementations often employ tools like the Automated Recommendation Tool (ART), which leverages machine learning to provide predictive models and recommendations for subsequent experimental designs [10].
Automated DBTL Workflow Architecture
The following diagram illustrates the information flow and key components in an automated DBTL pipeline:
Classic Antibiotic Discovery in Streptomycetes
Historical Significance and Workflow
Streptomycetes represent a historically significant platform for antibiotic production, having driven the golden age of antibiotics in the 1950s and 1960s. These Gram-positive bacteria are producers of a wide range of specialized metabolites with medicinal and industrial importance, including antibiotics, antifungals, and pesticides [11]. Traditional discovery approaches involved:
- Screening Natural Isolates: Researchers screened thousands of Streptomyces isolates from soil samples for antimicrobial activity.
- Mutation and Selection: Random mutagenesis using chemicals or UV radiation followed by screening for improved producers.
- Medium Optimization: Empirical testing of various carbon and nitrogen sources to enhance titers.
- Process Scale-up: Laboratory findings were translated to fermentation processes with minimal mechanistic understanding.
Despite the success of these approaches in producing first-generation antibiotics, technological advancements over the last two decades have revealed that only a fraction of the biosynthetic potential of Streptomycetes has been exploited [11]. Given the urgent need for new antibiotics due to the antimicrobial resistance crisis, there is renewed interest in applying engineering approaches like the DBTL cycle to explore and engineer this untapped potential.
DBTL Cycle Application to Streptomycetes
The contemporary application of the DBTL cycle to Streptomycetes engineering involves specialized approaches tailored to these actinobacteria:
- Design: Bioinformatics tools identify novel biosynthetic gene clusters and predict their functions. Pathway refactoring optimizes gene arrangement for heterologous expression.
- Build: Advanced genetic tools like CRISPR-Cas9 enable precise genome editing. Multiplex Automated Genome Engineering (MAGE) allows simultaneous modification of multiple genomic locations.
- Test: Analytical platforms (LC-MS/MS) characterize metabolite production and identify novel compounds. Cultivation platforms optimize production conditions.
- Learn: Multi-omics integration (genomics, transcriptomics, proteomics, metabolomics) reveals regulatory networks and metabolic bottlenecks.
This systematic approach has significantly accelerated the discovery and production of novel specialized metabolites from Streptomycetes, addressing the critical need for new antibiotics [11].
Contemporary Bioproduction: Automated DBTL Pipelines
Integrated Workflow Implementation
Modern biofoundries have implemented highly automated DBTL pipelines that significantly accelerate strain development cycles. These integrated systems demonstrate the power of contemporary bioproduction approaches:
Full Automation Integration: The pipeline runs from in silico selection of candidate enzymes through automated parts design, statistically guided pathway assembly, rapid testing, and rationalized redesign [9]. This integrated approach provides an iterative DBTL cycle underpinned by computational and laboratory automation.
Modular Design: The pipeline is constructed in a modular fashion, allowing laboratories to replace individual components while preserving overall principles and processes. This flexibility enables technology adoption as methods advance [9].
Compression of Design Space: Combinatorial design approaches generating thousands of possible configurations are reduced to tractable numbers using statistical methods like orthogonal arrays combined with Latin squares. This achieves compression ratios of 162:1 (2592 to 16 constructs), making comprehensive exploration feasible [9].
Case Study: Flavonoid Production in E. coli
The application of an automated DBTL pipeline to (2S)-pinocembrin production in E. coli demonstrates the efficiency of contemporary approaches:
- Initial Library Design: 2592 possible configurations were designed varying vector copy number, promoter strength, and gene order [9].
- DoE Reduction: Statistical reduction yielded 16 representative constructs [9].
- Production Range: Initial pinocembrin titers ranged from 0.002 to 0.14 mg L⁻¹ [9].
- Key Findings: Vector copy number had the strongest significant effect on production, followed by chalcone isomerase promoter strength [9].
- Second Cycle Optimization: Incorporating learnings from the first cycle improved production by 500-fold, achieving competitive titers up to 88 mg L⁻¹ [9].
This case study illustrates how iterative DBTL cycling with automation at every stage enables rapid pathway optimization, compressing development timelines that traditionally required years into weeks or months.
Quantitative Comparison of DBTL Approaches
Performance Metrics Across Applications
Table 1: Quantitative Performance of DBTL Applications in Metabolic Engineering
| Application | Host Organism | Target Compound | Production Improvement | Key Factors | Citation |
|---|---|---|---|---|---|
| Flavonoid Production | E. coli | (2S)-pinocembrin | 500-fold increase (to 88 mg L⁻¹) | Vector copy number, CHI promoter strength | [9] |
| Dopamine Production | E. coli | Dopamine | 2.6-6.6-fold improvement (69.03 ± 1.2 mg/L) | RBS engineering, GC content in SD sequence | [13] |
| Isoprenol Production | E. coli | Isoprenol | 23% improvement predicted | Machine learning recommendations from multi-omics | [10] |
Methodological Comparison
Table 2: Methodological Approaches in DBTL Implementation
| Methodological Aspect | Classic Approach | Contemporary Approach | Key Advantages |
|---|---|---|---|
| Design Methodology | Manual design based on literature | Automated computational tools (RetroPath, Selenzyme) | Comprehensive exploration, reduced bias |
| Build Technique | Manual cloning, restriction enzyme-based | Automated LCR assembly, robotics platform | Higher throughput, reduced human error |
| Test Capacity | Low-throughput analytics | UPLC-MS/MS with automated sample processing | Higher data quality, more replicates |
| Learn Mechanism | Empirical correlation | Machine learning (ART), statistical DoE | Predictive power, pattern recognition |
| Cycle Duration | Months to years | Weeks to months | Accelerated optimization |
Enabling Technologies and Methodologies
Computational and Analytical Tools
The implementation of effective DBTL cycles relies on sophisticated computational infrastructure and analytical tools:
Machine Learning Integration: ML methods like gradient boosting and random forest have demonstrated superior performance in the low-data regime common in early DBTL cycles. These methods show robustness to training set biases and experimental noise [14]. Automated recommendation algorithms leverage ML predictions to propose new strain designs, with studies showing that large initial DBTL cycles are favorable when the number of strains to be built is limited [14].
Multi-omics Data Integration: Tools like the Experiment Data Depot (EDD) serve as open-source repositories for experimental data and metadata. When combined with the Automated Recommendation Tool (ART) and Jupyter Notebooks, researchers can effectively store, visualize, and leverage synthetic biology data to enable predictive bioengineering [10].
Data Visualization: Advanced visualization techniques like GEM-Vis enable the dynamic representation of time-course metabolomic data within metabolic network maps. These visualization approaches allow researchers to observe metabolic state changes over time, facilitating new insights into network dynamics [15]. Effective visualization strategies are particularly crucial for interpreting complex untargeted metabolomics data throughout the analytical workflow [16].
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents and Solutions in DBTL Workflows
| Reagent/Solution | Composition/Type | Function in DBTL Workflow | Application Example |
|---|---|---|---|
| Minimal Medium | Defined carbon source, salts, trace elements | Controlled cultivation conditions | Dopamine production in E. coli [13] |
| SOC Medium | Tryptone, yeast extract, salts, glucose | Recovery after transformation | Cloning steps in strain construction [13] |
| Phosphate Buffer | KH₂PO₄/K₂HPO₄ at pH 7 | Reaction environment for cell-free systems | In vitro testing in knowledge-driven DBTL [13] |
| Reaction Buffer | Phosphate buffer with FeCl₂, vitamin B6, substrates | Supporting enzymatic activity | Crude cell lysate systems for pathway testing [13] |
| Trace Element Solution | Fe, Zn, Mn, Cu, Co, Ca, Mg salts | Providing essential micronutrients | Supporting robust cell growth in production [13] |
Advanced DBTL Methodologies
Knowledge-Driven DBTL Framework
A recent innovation in DBTL methodology is the knowledge-driven approach that incorporates upstream in vitro investigation:
Mechanistic Understanding: This approach uses cell-free protein synthesis (CFPS) systems and crude cell lysate systems to test enzyme expression levels and pathway functionality before implementing changes in living cells. This bypasses whole-cell constraints such as membranes and internal regulation [13].
RBS Engineering: Simplified ribosome binding site engineering modulates the Shine-Dalgarno sequence without interfering with secondary structures, enabling precise fine-tuning of relative gene expression in synthetic pathways [13].
Implementation Workflow: The knowledge-driven cycle begins with in vitro testing using crude cell lysate systems to assess different relative expression levels. Results are then translated to the in vivo environment through high-throughput RBS engineering, accelerating strain development [13].
This approach demonstrated its effectiveness in optimizing dopamine production in E. coli, where it achieved concentrations of 69.03 ± 1.2 mg/L, representing a 2.6-6.6-fold improvement over previous state-of-the-art production methods [13].
Multi-omics Integration and Visualization
The integration of multiple data types represents another significant advancement in DBTL capabilities:
Multi-omics Data Collection: Contemporary approaches leverage exponentially increasing volumes of multimodal data, including transcriptomics, proteomics, and metabolomics [10].
Synthetic Data Generation: Tools like the Omics Mock Generator (OMG) library produce biologically believable multi-omics data based on plausible metabolic assumptions. While not real, this synthetic data provides more realistic testing than randomly generated data, enabling rapid algorithm prototyping [10].
Dynamic Visualization: Methods like GEM-Vis create animated visualizations of time-course metabolomic data within metabolic network maps, using fill levels of nodes to represent metabolite amounts at each time point. These dynamic visualizations enable researchers to observe system behavior over time, facilitating new insights [15].
The relationship between data types, analytical methods, and visualization strategies can be represented as follows:
The evolution from classic antibiotic discovery to contemporary bioproduction represents a fundamental paradigm shift in metabolic engineering. The adoption of systematic DBTL cycles, enhanced by automation, machine learning, and multi-omics integration, has transformed the field from a trial-and-error discipline to a predictive engineering science. Where traditional approaches to antibiotic discovery in Streptomycetes relied on observational methods and empirical optimization, modern bioengineering leverages designed iterations with computational guidance to achieve precise metabolic outcomes.
This transition has profound implications for addressing contemporary challenges, from antimicrobial resistance to sustainable bioproduction. The continued refinement of DBTL methodologies—including knowledge-driven approaches, enhanced visualization techniques, and integrated biofoundries—promises to further accelerate the development of next-generation bacterial cell factories. As these technologies mature, they will undoubtedly expand the scope of accessible biological products and increase the efficiency of their production, ultimately strengthening the bioeconomy and addressing critical human needs.
Why Streptomycetes and E. coli are Prime Model Organisms for DBTL Applications
The Design-Build-Test-Learn (DBTL) cycle represents a systematic framework for accelerating microbial strain development in metabolic engineering. This iterative engineering paradigm involves designing genetic modifications, building engineered strains, testing their performance, and learning from the data to inform the next design cycle [1]. The DBTL framework has become central to synthetic biology and metabolic engineering, with automated biofoundries increasingly implementing these cycles to streamline development processes [3]. The power of the DBTL approach lies in its ability to continuously integrate experimental data to refine metabolic models and engineering strategies, thereby reducing the time and resources required to develop industrial-grade production strains.
This technical review examines why Escherichia coli and Streptomyces species have emerged as premier model organisms for implementing DBTL cycles in metabolic engineering. We analyze their complementary strengths, present experimental case studies, and provide detailed methodologies that demonstrate their utility in optimized bioproduction.
Escherichia coli as a DBTL Chassis
Physiological and Genetic Advantages
Escherichia coli possesses several inherent characteristics that make it exceptionally suitable for DBTL-based metabolic engineering. Its rapid growth rate (doubling times as short as 20 minutes), easy culture conditions, and metabolic plasticity enable quick iteration through DBTL cycles [17]. The wealth of biochemical and physiological knowledge accumulated over decades of research provides a strong foundation for rational design phases. Furthermore, E. coli's status as the best-characterized organism on Earth means researchers have access to an extensive collection of genetic tools and well-annotated genomic resources [17].
From a genetic manipulation perspective, E. coli exhibits high transformation efficiency and supports a wide variety of cloning vectors and engineering techniques. This genetic tractability significantly accelerates the "Build" phase of DBTL cycles. The availability of advanced techniques such as CRISPR-based genome editing, λ-Red recombineering, and MAGE (Multiplex Automated Genome Engineering) enables precise and rapid strain construction [17]. These attributes collectively make E. coli an ideal platform for high-throughput metabolic engineering approaches.
Case Study: Knowledge-Driven DBTL for Dopamine Production
A recent implementation of the knowledge-driven DBTL cycle in E. coli demonstrates the efficient optimization of dopamine production [3]. Researchers developed a highly efficient dopamine production strain capable of producing 69.03 ± 1.2 mg/L (equivalent to 34.34 ± 0.59 mg/g biomass), representing a 2.6 to 6.6-fold improvement over previous state-of-the-art production systems [3].
Table 1: Key Performance Metrics in E. coli DBTL Case Studies
| Product | Host Strain | Titer Achieved | Fold Improvement | Key Engineering Strategy |
|---|---|---|---|---|
| Dopamine | E. coli FUS4.T2 | 69.03 ± 1.2 mg/L | 2.6-6.6x | RBS engineering of hpaBC and ddc genes [3] |
| 1-Dodecanol | E. coli MG1655 | 0.83 g/L | >6x | Machine learning-guided protein profile optimization [18] |
| 2-Ketoisovalerate | E. coli W | 3.22 ± 0.07 g/L | N/A | Systems metabolic engineering with non-conventional substrate [19] |
Experimental Protocol: Knowledge-Driven DBTL with RBS Engineering
Design Phase: The dopamine pathway was designed to utilize L-tyrosine as a precursor, with conversion to L-DOPA catalyzed by the native E. coli 4-hydroxyphenylacetate 3-monooxygenase (HpaBC) and subsequent decarboxylation to dopamine by L-DOPA decarboxylase (Ddc) from Pseudomonas putida [3]. The key innovation was the upstream in vitro investigation using crude cell lysate systems to test different relative enzyme expression levels before in vivo implementation.
Build Phase: The engineering strategy employed high-throughput ribosome binding site (RBS) engineering to fine-tune the expression levels of hpaBC and ddc genes. The pET plasmid system served as a storage vector for heterologous genes, while the pJNTN plasmid was used for library construction. The production host E. coli FUS4.T2 was engineered for high L-tyrosine production through depletion of the transcriptional dual regulator TyrR and mutation of the feedback inhibition of chorismate mutase/prephenate dehydrogenase (tyrA) [3].
Test Phase: Strains were cultured in minimal medium containing 20 g/L glucose, 10% 2xTY medium, and appropriate supplements. Analytical methods quantified dopamine production and biomass formation, with high-throughput screening enabling rapid evaluation of multiple RBS variants [3].
Learn Phase: Data analysis revealed the impact of GC content in the Shine-Dalgarno sequence on RBS strength, providing mechanistic insights that informed subsequent design iterations. This knowledge-driven approach minimized the number of DBTL cycles required to achieve significant production improvements [3].
Machine Learning Integration in DBTL Cycles
The integration of machine learning with DBTL cycles has significantly enhanced E. coli metabolic engineering. In a notable example, researchers implemented two DBTL cycles to optimize dodecanol production using 60 engineered E. coli MG1655 strains [18]. The first cycle modulated ribosome-binding sites and acyl-ACP/acyl-CoA reductase selection in a pathway operon containing thioesterase (UcFatB1), reductase variants (Maqu2507, Maqu2220, or Acr1), and acyl-CoA synthetase (FadD). Measurement of both dodecanol titers and pathway protein concentrations provided training data for machine learning algorithms, which then suggested optimized protein expression profiles for the second cycle [18]. This approach generated a 21% increase in dodecanol titer in the second cycle, reaching 0.83 g/L – more than 6-fold greater than previously reported batch values for minimal medium [18].
Streptomycetes as a DBTL Chassis
Physiological and Metabolic Specialization
Streptomyces species are Gram-positive bacteria renowned for their exceptional capacity to produce diverse secondary metabolites. These soil-dwelling bacteria possess complex genomes (8-10 MB with >70% GC content) encoding numerous biosynthetic gene clusters (BGCs) – approximately 36.5 per genome on average [20] [21]. Their natural physiological specialization for secondary metabolite production includes sophisticated regulatory networks, extensive precursor supply pathways, and specialized cellular machinery for compound secretion and self-resistance [21].
Streptomycetes exhibit a complex developmental cycle involving mycelial growth and sporulation, processes intrinsically linked to their secondary metabolism [21]. This inherent metabolic complexity provides a favorable cellular environment for the heterologous production of complex natural products, particularly large bioactive molecules such as polyketides and non-ribosomal peptides that often challenge other production hosts due to folding, solubility, or post-translational modification requirements [21].
Case Study: Systems Metabolic Engineering of Streptomyces Coelicolor
Genome-scale metabolic models (GSMMs) have played a crucial role in advancing DBTL applications in Streptomycetes. The iterative development of S. coelicolor models – from iIB711 to iMA789, iMK1208, and the most recent iAA1259 – demonstrates how increasingly sophisticated computational tools enhance DBTL efficiency [22]. Each model iteration has incorporated expanded reaction networks, improved gene-protein-reaction relationships, and updated biomass composition data, leading to progressively more accurate predictive capabilities.
Table 2: Streptomyces DBTL Tools and Applications
| Tool Category | Specific Tools/Examples | Function in DBTL Cycle | Reference |
|---|---|---|---|
| Genetic Tools | pIJ702, pSETGUS, pIJ12551 | Cloning and heterologous expression [20] [23] | |
| Computational Models | iAA1259 GSMM | Predicting metabolic fluxes and engineering targets [22] | |
| Automation Tools | ActinoMation (OT-2 platform) | High-throughput conjugation workflow [23] | |
| Database Resources | StreptomeDB | Natural product database for target identification [20] |
The iAA1259 model represents a significant advancement, incorporating multiple updated pathways including polysaccharide degradation, secondary metabolite biosynthesis (e.g., yCPK, gamma-butyrolactones), and oxidative phosphorylation reactions [22]. Model validation demonstrated substantially improved dynamic growth predictions, with iAA1259 achieving just 5.3% average absolute error compared to 37.6% with the previous iMK1208 model [22]. This enhanced predictive capability directly supports more effective Design phases in DBTL cycles.
Experimental Protocol: Automated Conjugation Workflow
A key limitation in Streptomyces DBTL cycles has been the laborious and slow transformation protocols. Recent work has addressed this bottleneck through automation with the ActinoMation platform, which implements a semi-automated medium-throughput workflow for introducing recombinant DNA into Streptomyces spp. using the open-source Opentrons OT-2 robotics platform [23].
The methodology involves:
- Strain Preparation: Preparation of donor E. coli ET12567/pUZ8002 strains carrying the desired plasmid and recipient Streptomyces spores.
- Automated Conjugation: The robotic platform performs the conjugation protocol, including mixing of donor and recipient cells, plating on appropriate media, and incubation.
- Selection and Analysis: Exconjugants are selected using appropriate antibiotics, with efficiency rates varying by strain and plasmid combination [23].
Validation across multiple Streptomyces species (S. coelicolor M1152 and M1146, S. albidoflavus J1047, and S. venezuelae DSM40230) demonstrated conjugation efficiencies ranging from 1.21×10⁻⁵ for S. albidoflavus with pSETGUS to 6.13×10⁻² for S. venezuelae with pIJ12551 [23]. This automated approach enables scalable DBTL implementation without sacrificing efficiency.
Comparative Analysis and Future Perspectives
Complementary Strengths in DBTL Applications
E. coli and Streptomycetes offer complementary advantages that make them suitable for different metabolic engineering applications within the DBTL framework:
E. coli excels in:
- Rapid DBTL iteration due to fast growth and well-established high-throughput tools
- Precise genetic control with extensive characterized parts (promoters, RBSs)
- Superior performance for products requiring precursors from central metabolism
- Advanced machine learning integration with rich historical data [3] [17] [18]
Streptomycetes excel in:
- Production of complex secondary metabolites requiring specialized tailoring enzymes
- Native capacity for antibiotic production and self-resistance
- Superior protein secretion capabilities benefiting downstream processing
- Natural enzymatic diversity for biotransformations [20] [21]
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagent Solutions for DBTL Applications
| Reagent/Resource | Function | Example Strains/Plasmids |
|---|---|---|
| E. coli Production Strains | Metabolic engineering chassis | FUS4.T2 (high L-tyrosine), MG1655 (dodecanol production), W (2-KIV production) [3] [18] [19] |
| Streptomyces Production Strains | Heterologous expression hosts | S. coelicolor M1152/M1146, S. albidoflavus J1047, S. venezuelae DSM40230 [23] |
| Cloning Vectors (E. coli) | Genetic manipulation | pET system (gene storage), pJNTN (library construction) [3] |
| Cloning Vectors (Streptomyces) | Heterologous expression | pSETGUS, pIJ12551, pIJ702 [20] [23] |
| Database Resources | Design phase guidance | StreptomeDB (natural products), GSMM models (iAA1259) [20] [22] |
Emerging Trends and Future Directions
The future of DBTL applications in both model organisms points toward increased integration of machine learning algorithms, automation, and multi-omics data integration. For E. coli, research focuses on expanding substrate utilization to non-conventional carbon sources [17] [19] and enhancing predictive models through deeper mechanistic understanding [1]. For Streptomycetes, efforts concentrate on developing more efficient genetic tools [21] [23] and leveraging genomic insights to unlock their extensive secondary metabolite potential [20] [22].
A particularly promising direction is the use of simulated DBTL cycles for benchmarking machine learning methods, as demonstrated in recent research showing that gradient boosting and random forest models outperform other methods in low-data regimes [1]. This approach enables optimization of DBTL strategies before costly experimental implementation, potentially accelerating strain development for both organism classes.
E. coli and Streptomycetes each occupy distinct but complementary niches as model organisms for DBTL applications in metabolic engineering. E. coli provides a streamlined platform for rapid iteration and high-throughput engineering, particularly valuable for products aligned with its central metabolism. Streptomycetes offer specialized capabilities for complex natural product synthesis, leveraging their native metabolic sophistication. The continued development of genetic tools, computational models, and automated workflows for both organisms will further enhance their utility in the DBTL framework, accelerating the development of microbial cell factories for sustainable bioproduction across diverse applications.
Visual Appendix: DBTL Workflow Diagrams
Diagram 1: The DBTL Cycle in Metabolic Engineering. This iterative framework forms the foundation for modern strain development, with each phase generating outputs that inform subsequent cycles.
Diagram 2: Comparative Strengths of E. coli and Streptomycetes as DBTL Chassis. Each organism offers specialized capabilities that make them suitable for different metabolic engineering applications.
Implementing the DBTL Cycle: Tools, Automation, and Real-World Applications
The Design-Build-Test-Learn (DBTL) cycle is a cornerstone framework in metabolic engineering and synthetic biology, enabling the systematic development of microbial strains for chemical production [24]. Within this iterative process, the Design phase serves as the critical foundational stage where theoretical strategies and precise genetic blueprints are formulated before physical construction begins. This phase has been transformed by computational tools, allowing researchers to move from intuitive guesses to data-driven designs [25].
This technical guide examines the core components of the Design phase, focusing on computational methods for strain design and the subsequent translation of these designs into actionable DNA assembly protocols. We will explore the algorithms and software tools that predict effective genetic modifications, the standardization of genetic parts, and the detailed planning of assembly strategies that ensure successful transition to the Build phase [26]. The precision achieved during Design directly determines the efficiency of the entire DBTL cycle, reducing costly iterations and accelerating the development of high-performance production strains.
Computational Methods for Strain Design
Computational strain design leverages genome-scale metabolic models and sophisticated algorithms to predict genetic modifications that enhance the production of target compounds. These tools identify which gene deletions, additions, or regulatory changes will redirect metabolic flux toward desired products while maintaining cellular viability [25].
Key Computational Approaches and Tools
Table 1: Computational Tools for Metabolic Engineering Strain Design
| Tool Name | Primary Function | Methodology | Application Example |
|---|---|---|---|
| RetroPath [9] | Pathway discovery | Analyzes metabolic networks to identify novel biological routes to target chemicals | Automated enzyme selection for flavonoid production pathways in E. coli |
| Selenzyme [9] | Enzyme selection | Selects suitable enzymes for specified biochemical reactions | Selecting enzymes for (2S)-pinocembrin pathway from Arabidopsis thaliana and Streptomyces coelicolor |
| OptKnock [25] | Gene knockout identification | Uses constraint-based modeling to couple growth with product formation | Predicting gene deletions to overproduce metabolites in yeast |
| Protein MPNN [27] | Protein design | AI-driven protein sequence design for creating novel enzymes | Generating protein libraries for biofoundry services |
These tools address different aspects of the design challenge. Pathway design tools like RetroPath explore what compounds can be made biologically using native, heterologous, or enzymes with broad specificity [25] [9]. Strain optimization algorithms then determine the genetic modifications needed to improve production titers, yield, and productivity for the designed pathways. Recent advancements have focused on improving runtime performance to identify more complex metabolic engineering strategies and incorporating kinetic considerations to improve prediction accuracy [25].
Implementing Computational Designs
The transition from computational prediction to implementable design requires careful consideration of genetic context. The PartsGenie software facilitates this transition by designing reusable DNA parts with simultaneous optimization of bespoke ribosome-binding sites and enzyme coding regions [9]. These tools enable the creation of combinatorial libraries of pathway designs, which can be statistically reduced using Design of Experiments (DoE) methodologies to manageable sizes for laboratory construction and screening [9].
For example, in a project aiming to produce the flavonoid (2S)-pinocembrin in E. coli, researchers designed a combinatorial library covering 2,592 possible configurations varying vector copy number, promoter strengths, and gene orders. Through DoE, this was reduced to 16 representative constructs, achieving a 162:1 compression ratio while maintaining the ability to identify significant factors affecting production [9].
DNA Assembly Protocol Design
Once a strategic strain design has been established computationally, the focus shifts to designing the physical DNA assembly protocols that will bring the design to life. This process involves selecting appropriate assembly methods, designing genetic parts with correct specifications, and generating detailed experimental protocols.
DNA Assembly Methodologies
Table 2: Common DNA Assembly Methods in Metabolic Engineering
| Method | Key Feature | Advantages | Common Applications |
|---|---|---|---|
| Golden Gate Assembly [28] | Type IIS restriction enzyme-based | Modularity, one-pot reaction, standardization | Pathway construction, toolkit development (e.g., YaliCraft) |
| Gibson Assembly [29] | Isothermal assembly | Seamless, single-reaction, no sequence constraints | Plasmid construction, multi-fragment assembly |
| Ligase Cycling Reaction (LCR) [9] | Oligonucleotide assembly | High efficiency, error-free, customizable | Pathway library construction, automated workflows |
| CRISPR/Cas9 Integration [28] | Genome editing | Marker-free integration, chromosomal insertion | Direct genomic integration, multiplexed editing |
Modern metabolic engineering projects often employ hierarchical modular cloning systems that combine these methods. For instance, the YaliCraft toolkit for Yarrowia lipolytica employs Golden Gate assembly as its primary method, organized into seven individual modules that can be applied in different combinations to enable complex strain engineering operations [28]. The toolkit includes 147 plasmids and enables operations such as gene overexpression, gene disruption, promoter library screening, and easy redirection of integration events to different genomic loci.
Protocol Design Considerations
When designing DNA assembly protocols, several technical factors must be addressed:
- Restriction enzyme selection: For Golden Gate assembly, careful selection of Type IIS restriction enzymes is crucial to ensure compatibility and avoid internal cut sites [26].
- Homology arm design: For CRISPR/Cas9 integration, homology arms typically require 500-1000bp flanking sequences for efficient homologous recombination in yeast systems [28].
- Parts compatibility: Automated software tools can verify compatibility among DNA fragments, considering factors such as GC content, secondary structure, and repetitive elements [26].
- Inventory optimization: Advanced design platforms can optimize the use of existing lab inventory, reducing additional DNA synthesis orders and associated costs [26].
The design of assembly protocols has been greatly enhanced by specialized software that automatically generates detailed experimental protocols based on the desired genetic construct. These platforms can select appropriate cloning methods, design optimal fragment arrangements, and even generate robotic worklists for automated liquid handling systems [26] [9].
Integrated Workflows and Visualization
The complete Design phase integrates computational strain design with DNA assembly protocol generation through a structured workflow. The following diagram illustrates this integrated process:
Design Workflow: The integrated process from target compound to DNA assembly protocol.
Design Phase in Biofoundry Operations
In automated biofoundries, the Design phase is formalized through standardized workflows and unit operations to ensure reproducibility and interoperability. According to the proposed abstraction hierarchy for biofoundry operations, the Design phase encompasses several specific workflows [27]:
- WB010: DNA Oligomer Assembly - Designing oligonucleotides for gene synthesis
- WB021: Metabolic Model Simulation - Using computational models to predict strain behavior
- WB030: Genetic Circuit Design - Designing regulatory systems and genetic constructs
- WB040: Parts Engineering - Creating and characterizing standard biological parts
These workflows are composed of specific unit operations, which represent the smallest executable tasks in the design process. For example, the DNA Oligomer Assembly workflow can be decomposed into 14 distinct unit operations including oligonucleotide design, sequence optimization, and synthesis planning [27].
The Scientist's Toolkit: Essential Research Reagents
Implementing the Design phase requires both computational tools and physical research reagents. The following table details essential materials and their functions in computational strain design and DNA assembly protocol development.
Table 3: Essential Research Reagents and Resources for the Design Phase
| Category | Item | Function | Example/Specification |
|---|---|---|---|
| Software Platforms | TeselaGen [26] | End-to-end DBTL platform supporting DNA assembly protocol generation | Cloud or on-premises deployment |
| JBEI-ICE [9] | Repository for biological parts, designs, and samples | Open-source registry platform | |
| DNA Design Tools | PartsGenie [9] | Automated design of reusable DNA parts | Optimizes RBS and coding sequences |
| PlasmidGenie [9] | Automated generation of assembly recipes and robotics worklists | Outputs LCR assembly instructions | |
| Strain Design Tools | RetroPath2.0 [9] | Automated pathway design from target compound | Explores metabolic space for novel routes |
| Selenzyme [9] | Enzyme selection for specified reactions | Recommends enzymes based on sequence and structure | |
| DNA Assembly Kits | Golden Gate Toolkits [28] | Modular cloning systems for specific organisms | YaliCraft (Y. lipolytica), Yeast Toolkit (S. cerevisiae) |
| CRISPR/Cas9 Systems [28] | Marker-free genomic integration | Cas9 helper plasmids, gRNA constructs | |
| DNA Providers | Twist Bioscience [26] | High-quality DNA synthesis | Custom gene fragments, oligo pools |
| IDT [26] | DNA synthesis and assembly reagents | gBlocks, custom primers |
This toolkit enables researchers to transition seamlessly from computational designs to executable protocols. For instance, the integration between TeselaGen's design platform and DNA synthesis providers like Twist Bioscience allows for direct ordering of designed sequences, creating a streamlined workflow from digital design to physical DNA [26].
The Design phase represents a critical integration point between computational prediction and practical implementation in metabolic engineering. Through sophisticated algorithms for strain design and meticulous planning of DNA assembly protocols, this phase sets the trajectory for successful DBTL cycles. The continued development of more predictive computational models, standardized biological parts, and automated design workflows will further accelerate the engineering of microbial cell factories for sustainable chemical production.
As the field advances, the incorporation of machine learning and artificial intelligence promises to enhance the predictive power of design tools, potentially reducing the number of DBTL iterations required to achieve production targets [26] [30]. Furthermore, the standardization of design workflows across biofoundries will improve reproducibility and collaboration, ultimately advancing the entire field of metabolic engineering.
The Design-Build-Test-Learn (DBTL) cycle is a foundational framework in synthetic biology and metabolic engineering for systematically developing and optimizing biological systems [12]. Within this iterative process, the Build phase is the critical step where designed genetic constructs are physically assembled and introduced into a host organism to create the engineered strains ready for testing [31]. This phase has traditionally been a major bottleneck in metabolic engineering due to the time-consuming and labor-intensive nature of traditional genetic manipulation techniques [32]. The integration of CRISPR-Cas9 systems and automated liquid handlers has revolutionized the Build phase, enabling unprecedented throughput, precision, and efficiency in strain construction [31]. This technical guide examines how these technologies synergize to accelerate the creation of genetic variants, thereby transforming our capability to engineer microbial cell factories for producing biofuels, pharmaceuticals, and specialty chemicals [32] [31].
CRISPR-Cas9 Tools for Genetic Perturbation
The CRISPR-Cas9 system provides a programmable platform for diverse genetic manipulations. Its core components—a Cas nuclease and a guide RNA (gRNA)—can be engineered or repurposed to achieve specific genetic outcomes [33]. The table below summarizes the key CRISPR modalities used in high-throughput genetic engineering.
Table 1: CRISPR-Cas9 Modalities for Genetic Engineering
| CRISPR Modality | Key Components | Mechanism of Action | Primary Application in Build Phase |
|---|---|---|---|
| CRISPR Knockout (CRISPRd) | Cas9 nuclease, sgRNA | Introduces double-strand breaks repaired by error-prone non-homologous end-joining (NHEJ), leading to indel mutations and gene knockouts [33]. | Permanent disruption of gene function [34]. |
| CRISPR Interference (CRISPRi) | catalytically dead Cas9 (dCas9) fused to repressor domains (e.g., KRAB), sgRNA [33] [35]. | Binds to DNA without cutting, blocking transcription initiation or elongation via steric hindrance or chromatin modification [33] [35]. | Reversible, tunable gene downregulation [33] [34]. |
| CRISPR Activation (CRISPRa) | dCas9 fused to activator domains (e.g., VP64, p65, Rta), sgRNA [33] [35]. | Recruits transcriptional machinery to promoter regions to enhance gene expression [33]. Systems include SunTag, SAM, and VPR [35]. | Targeted gene upregulation or activation of silent pathways [34] [35]. |
| Base Editing | Cas9 nickase (nCas9) fused to deaminase enzymes, sgRNA [31]. | Mediates direct chemical conversion of one DNA base to another (e.g., C to T) without double-strand breaks or donor templates [31]. | High-efficiency point mutations for functional studies or correction [31]. |
| CRISPR-Mediated HDR | Cas9 nuclease, sgRNA, donor DNA template [31]. | Uses homology-directed repair (HDR) with an exogenous donor template to introduce precise edits, insertions, or deletions [31]. | Precise gene insertion, tag addition, or single-nucleotide replacement [31]. |
High-Throughput Library Construction
A principal application of CRISPR-Cas9 in the Build phase is the generation of comprehensive genetic libraries for functional genomics and pathway optimization. These libraries consist of pooled gRNA-encoding plasmids that enable simultaneous perturbation of thousands of genomic targets [32] [33].
Table 2: Types of Genetic Libraries for High-Throughput Screening
| Library Type | Description | Perturbation Scale | Proof-of-Concept Application |
|---|---|---|---|
| Genome-Wide Knockout (CRISPRd) | Library of sgRNAs targeting constitutive exons of all genes to create frameshift mutations [33] [34]. | Genome-wide coverage with ~4 sgRNAs per gene on average [34]. | Identification of essential genes and determinants of drug resistance [33]. |
| CRISPRi/a Libraries | sgRNAs designed to bind promoter regions for repression (CRISPRi) or activation (CRISPRa) of all genes [33] [34]. | Designed with ~6 sgRNAs per gene for broad coverage [34]. | Discovery of genetic modifiers for complex phenotypes like furfural tolerance [34]. |
| Multifunctional Libraries (e.g., MAGIC) | Combines CRISPRd, CRISPRi, and CRISPRa in a single system using orthogonal Cas proteins [34]. | One of the most comprehensive libraries in yeast, covering gain-of-function, reduction-of-function, and loss-of-function [34]. | Engineering complex phenotypes like protein surface display through synergistic multi-gene perturbations [34]. |
| Oligo-Mediated Libraries | Utilizes array-synthesized oligonucleotide pools as templates for recombineering or direct cloning [32]. | Libraries containing >10^6 variants can be generated within one week [32]. | Fine-tuning metabolic pathways through ribosomal binding site (RBS) engineering [32]. |
Experimental Protocol: Building a Genome-Wide CRISPR Knockout Library
The following protocol details the key steps for constructing a genome-wide CRISPR knockout library, adaptable for other CRISPR modalities [33] [34]:
gRNA Library Design and Oligo Synthesis:
- Select 4-6 target-specific 20-nucleotide guide sequences for each gene in the genome. Prioritize early constitutive exons to maximize the probability of gene disruption [33] [34].
- Design oligos with the structure:
5'-Adapter-Guide Sequence-gRNA Scaffold-Adapter-3'. Exclude sequences with polyT or polyG tracts and internal BsaI restriction sites [34]. - Synthesize the oligo pool in an arrayed format.
Library Cloning:
- Amplify the pooled oligonucleotides via PCR to add necessary flanking sequences for cloning.
- Digest the amplified PCR product and the recipient gRNA expression plasmid with the appropriate restriction enzymes (e.g., BsaI for Golden Gate Assembly) [34].
- Ligate the digested insert and vector. The assembly efficiency can be estimated by genotyping random clones (e.g., 14 colonies), with near 100% efficiency achievable [34].
Transformation and Library Validation:
- Transform the ligated plasmid library into competent E. coli cells to achieve a transformation count that significantly exceeds the library diversity (e.g., >1000x coverage) to ensure full representation.
- Harvest the plasmid library from the bacteria.
- Validate the library by next-generation sequencing (NGS) of the gRNA inserts to confirm coverage and uniformity. A well-constructed library should have correct guide sequences for >99.9% of target genes [34].
Delivery into Host Cells:
- Introduce the validated plasmid library into the host organism (e.g., yeast or mammalian cells) via high-efficiency transformation methods, often using viral transduction (lentivirus for mammalian cells) [33] [35].
- For CRISPRi/a, the host cell must stably express dCas9 fused to the appropriate effector domain (repressor or activator) [33] [35].
Integration of Automated Liquid Handlers
Automation is the force multiplier that transforms CRISPR library technology into a truly high-throughput Build process. Automated liquid handlers execute repetitive pipetting tasks with superior precision, speed, and reproducibility compared to manual methods [36].
Key Applications in the Build Phase
- High-Throughput Cloning: Automated systems can set up thousands of parallel restriction-ligation reactions or Golden Gate assemblies, drastically reducing hands-on time and variability in library construction [36].
- Transformation and Colony Picking: Robots can efficiently process transformation reactions, plate out colonies, and pick thousands of individual clones for screening, eliminating a major manual bottleneck [12] [36].
- Culture Inoculation and Maintenance: Automated systems can inoculate cultures in 96- or 384-well plates and perform serial dilutions or media exchanges to maintain library cultures during outgrowth, ensuring uniform culture conditions [36].
Experimental Protocol: Automated Library Cloning Workflow
This protocol outlines a automated workflow for cloning a CRISPR library:
Reagent Setup:
- Dilute the synthesized oligo pool to a working concentration in a 96-well source plate.
- Prepare a master mix containing PCR reagents (polymerase, dNTPs, buffer) in a reservoir.
- Dispense the recipient vector plasmid into a separate reservoir.
Automated PCR Setup:
- Program the liquid handler to transfer the oligo pool from the source plate into a 96-well PCR plate.
- Dispense the PCR master mix into each well of the PCR plate.
- Seal the plate and transfer it to a thermal cycler for amplification.
Automated Golden Gate Assembly:
- Program the robot to mix the purified PCR product (insert), the recipient vector, restriction enzyme (e.g., BsaI), ligase, and buffer in a new reaction plate.
- The assembly reaction is then incubated in a thermal cycler.
Automated Transformation Preparation:
- Aliquot competent E. coli cells into a chilled deep-well plate using the liquid handler.
- Transfer the assembly reaction into the competent cells for transformation.
- After heat shock, add recovery medium and incubate with shaking. The culture is then transferred to agar plates for colony growth.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for High-Throughput CRISPR Build Phase
| Reagent / Solution | Function | Application Notes |
|---|---|---|
| Array-Synthesized Oligo Pools | Source of sequence diversity for generating gRNA libraries [32] [34]. | Designed with flanking adapters for efficient cloning. Quality control via NGS is critical [34]. |
| Cas9/dCas9 Expression Constructs | Provides the CRISPR effector protein (nuclease, repressor, or activator) in the host cell [33] [35]. | For CRISPRi/a, dCas9 is fused to transcriptional regulator domains like KRAB (repressor) or VP64/p65 (activator) [33] [35]. |
| gRNA Expression Vectors | Plasmid backbone for expressing sgRNAs from a Pol III promoter (e.g., U6) in the host [33]. | Must be compatible with the chosen Cas9/dCas9 ortholog and the host's genetic system. |
| Restriction Enzymes & Ligases | Enzymatic assembly of gRNA expression cassettes into the vector backbone [34]. | Type IIS enzymes (e.g., BsaI) are preferred for Golden Gate Assembly as they enable seamless and modular construction [34]. |
| High-Efficiency Competent Cells | Cloning and propagation of plasmid libraries in E. coli [37]. | Requires high transformation efficiency (>10^9 CFU/μg) to ensure full library representation. |
| Lentiviral Packaging System | Production of viral particles for delivery of CRISPR components into hard-to-transfect cells (e.g., mammalian cells) [33] [35]. | Essential for pooled screening in mammalian systems; allows for stable integration. |
| Liquid Handler Consumables | Tips, plates, and reservoirs for automated liquid handling. | Use of low-adhesion tips and plates minimizes sample loss and cross-contamination in high-throughput workflows. |
The integration of CRISPR-Cas9 technologies with automated liquid handling systems has decisively addressed the Build phase as a historical bottleneck in the DBTL cycle [31]. This powerful synergy enables the rapid and precise construction of highly complex genetic libraries—including knockouts, knockdowns, and activations—at an unprecedented scale [32] [34]. The standardized, automated protocols ensure reproducibility and speed, allowing researchers to generate thousands of engineered strains in a fraction of the time required by manual methods [36]. By transforming the Build phase into a high-throughput, data-rich process, these advanced tools empower metabolic engineers to more effectively explore vast genetic landscapes, accelerating the development of robust microbial cell factories for a sustainable bioeconomy.
In the context of the Design-Build-Test-Learn (DBTL) cycle for metabolic engineering, the Test phase is where engineered biological systems are rigorously evaluated. It transforms constructed genetic designs into quantifiable data, forming the critical feedback loop that drives the entire iterative engineering process. This phase leverages high-throughput phenotyping—the comprehensive, automated assessment of complex traits—to generate the robust datasets necessary for informed learning and redesign.
The Role of High-Throughput Phenotyping in the DBTL Cycle
High-throughput phenotyping (HTP) addresses a fundamental bottleneck in biotechnology and metabolic engineering. Traditional phenotyping methods are often destructive, labor-intensive, and low-throughput, unable to keep pace with modern capabilities for generating large numbers of engineered strains or plant varieties [38]. The DBTL framework, a cornerstone of synthetic biology, relies on testing multiple permutations of a design to achieve a desired outcome, such as optimized production of a valuable compound [12]. HTP provides the scalable, data-rich "Test" phase that makes rapid DBTL cycling possible.
Within the DBTL cycle, the Test phase is responsible for:
- Generating Performance Data: Quantifying the output of engineered systems, such as metabolite titers, growth rates, or functional characteristics.
- Identifying Bottlenecks: Revealing limitations in engineered pathways by measuring intermediates and overall flux.
- Providing Ground Truth: Supplying the high-quality empirical data required for the subsequent "Learn" phase, where statistical and machine learning models are applied to inform the next design cycle [9] [3].
High-Throughput Phenotyping Technologies and Platforms
HTP utilizes a suite of non-invasive sensors and automated platforms to collect temporal and spatial data on physiological, morphological, and biochemical traits. These platforms operate at multiple scales, from microscopic analysis to field-level evaluation.
Phenotyping Platforms Across Scales
The table below summarizes key HTP platforms and the types of traits they record.
Table 1: Overview of High-Throughput Phenotyping Platforms
| Platform Name | Scale | Primary Traits Recorded | Application Example |
|---|---|---|---|
| LemnaTec 3D Scanalyzer [38] | Ground-based | Salinity tolerance traits | Screening rice for salt tolerance [38] |
| PHENOVISION [38] | Ground-based | Drought stress and recovery responses | Monitoring maize response to water deficit [38] |
| PlantScreen [38] | Ground-based | Drought tolerance traits | Analyzing abiotic stress responses in rice [38] |
| PhenoSelect [39] | Lab-based (Microbial) | Photosynthetic efficiency, growth rate, cell size | Profiling microalgae for biofuel applications [39] |
| HyperART [38] | Ground-based | Leaf chlorophyll content, disease severity | Quantifying disease severity in barley and maize [38] |
| Unmanned Aerial Vehicles (UAVs) [38] | Aerial | Biomass yield, plant health, abiotic stress | Field-based assessment of crop health and yield [38] |
Core Analytical Techniques in Metabolic Phenotyping
The platforms above are integrated with sophisticated analytical instruments to provide deep metabolic insights. Key technologies include:
- Mass Spectrometry (MS): Often coupled with liquid chromatography (LC-MS/MS), this technique is a workhorse for the precise identification and quantification of target metabolites and pathway intermediates. In automated DBTL pipelines, it enables rapid, quantitative screening of compounds like flavonoids or alkaloids from microbial cultures [9].
- Fluorometry and Spectroscopy: These are used for non-destructive, real-time monitoring of physiological status. For example, chlorophyll fluorescence can report on photosynthetic efficiency and stress responses in plants and microalgae [38] [39].
- Seahorse XF Analyzer: This instrument simultaneously measures the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) of live cells, providing a real-time window into mitochondrial function and cellular energy metabolism [40].
- Flow Cytometry: This technology allows for the analysis of physical and chemical characteristics of individual cells within a population. It is invaluable for assessing cell size, complexity, and fluorescence-based markers in a high-throughput manner [39].
- Nuclear Magnetic Resonance (NMR): NMR is used for the non-destructive determination of body composition in small animals and can provide detailed information on metabolite structure and abundance [40].
Data Analysis: Integrating Machine and Deep Learning
The application of HTP generates massive, complex datasets. Machine Learning (ML) and Deep Learning (DL) provide the necessary computational tools to extract meaningful biological insights from this data deluge [38].
- Machine Learning: ML approaches, such as supervised learning for classification (e.g., healthy vs. diseased plants) and unsupervised learning for pattern discovery, can handle large amounts of data effectively. However, they often require significant manual effort for "feature engineering"—identifying and quantifying the relevant parameters for analysis [38].
- Deep Learning: DL has emerged as a powerful subset of ML that bypasses the need for manual feature engineering. Convolutional Neural Networks (CNNs), a primary DL architecture, are now the state-of-the-art for image-based phenotyping tasks such as image classification, object recognition, and segmentation. This allows for the automatic learning of hierarchical features directly from raw sensor data, such as images from drones or ground-based platforms [38].
Experimental Protocols for High-Throughput Testing
The following protocols illustrate how HTP is implemented in practice for different biological systems.
Protocol: High-Throughput Screening of Microbial Metabolite Production
This protocol is adapted from automated DBTL pipelines for producing fine chemicals in E. coli [9].
Objective: To quantitatively screen a library of engineered E. coli strains for the production of a target compound (e.g., pinocembrin) in a 96-deepwell plate format.
Materials:
- Library of engineered E. coli strains in 96-deepwell plates.
- Sterile growth medium with appropriate inducers and antibiotics.
- Automated liquid handling system.
- Centrifuge compatible with microplates.
- Automated metabolite extraction system.
- UPLC-MS/MS (Ultra-Performance Liquid Chromatography coupled with Tandem Mass Spectrometry) system.
Procedure:
- Inoculation and Growth: Use an automated liquid handler to inoculate sterile growth medium in 96-deepwell plates with the engineered strains. Seal the plates with breathable seals.
- Incubation: Incubate the plates in a controlled environment shaker at the optimal temperature for growth and protein expression.
- Induction: At the target cell density, automatically add inducer (e.g., IPTG) to trigger expression of the metabolic pathway.
- Harvesting: After a defined production period, centrifuge the plates to pellet cells.
- Metabolite Extraction: Automatically extract metabolites from the cell pellet or supernatant using a standardized solvent system (e.g., methanol or acetonitrile).
- Analysis: Inject the extracted samples directly into the UPLC-MS/MS system for separation and quantification of the target compound and key pathway intermediates.
- Data Processing: Use custom-developed scripts (e.g., in R or Python) for automated data extraction, peak integration, and titer calculation [9].
Protocol: Phenotyping Abiotic Stress in Plants
Objective: To non-destructively assess drought stress responses in a cereal crop (e.g., maize or wheat) using aerial and ground-based platforms.
Materials:
- Experimental field plots with controlled drought stress conditions.
- Unmanned Aerial Vehicle (UAV) equipped with multispectral or hyperspectral sensors.
- Ground-based phenotyping platform (e.g., LemnaTec Scanalyzer).
- Data storage and processing infrastructure.
Procedure:
- Experimental Design: Establish a randomized block design with replicated plots of different genotypes under well-watered and drought-stressed conditions.
- Temporal Data Acquisition:
- Aerial: Fly the UAV over the field plots at regular intervals throughout the growing season. Capture high-resolution multispectral images (e.g., RGB, Near-Infrared, Red Edge).
- Ground-based: Use the ground platform to capture higher magnification images of individual plants, including visible, fluorescence, and thermal imaging.
- Data Processing:
- Stitch aerial images into orthomosaics for the entire field.
- Extract vegetation indices (e.g., NDVI for biomass, PRI for water stress) for each plot or plant.
- Trait Analysis: Use machine learning or deep learning models to analyze the image data and derive traits such as:
- Canopy cover and biomass (from RGB and NIR).
- Chlorophyll content (from spectral indices).
- Canopy temperature (from thermal imaging, an indicator of water stress).
- Data Integration: Correlate the HTP-derived traits with final yield data and physiological measurements to validate the phenotyping approach [38] [41].
Visualizing Workflows and Pathways
The following diagrams illustrate the logical flow of the Test phase and a specific metabolic pathway analyzed within it.
Test Phase Workflow
Dopamine Biosynthesis Pathway
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key Research Reagent Solutions for High-Throughput Phenotyping
| Item / Solution | Function in the Test Phase | Application Example |
|---|---|---|
| Cell Lysis Reagents | Breaks open cells to release intracellular metabolites for analysis. | Used in crude cell lysate systems for in vitro pathway testing prior to full in vivo strain engineering [3]. |
| Stable Isotope Labels | Enables tracking of carbon and nutrient flux through metabolic pathways. | Used with LC-MS to perform 13C-metabolic flux analysis and identify pathway bottlenecks. |
| Specialized Growth Media | Provides controlled nutritional environment for consistent culturing. | Minimal media with defined carbon sources for microbial production [3]; hydroponic systems for controlled plant stress studies. |
| Spectral Probes & Dyes | Binds to specific cellular components for fluorescence-based detection. | Viability stains, membrane potential dyes for flow cytometry; stains for root structure imaging. |
| Enzyme Assay Kits | Provides optimized reagents for quantifying specific enzyme activities. | Measuring the activity of key pathway enzymes (e.g., dehydrogenases, kinases) in a high-throughput microplate format. |
| Multiplex Assay Kits | Allows simultaneous measurement of dozens of analytes from a single sample. | Quantifying panels of cytokines, hormones, or other signaling molecules from serum, plasma, or tissue extracts [40]. |
The Test phase, powered by high-throughput phenotyping, is the data engine of the DBTL cycle. The integration of automated platforms, advanced analytical techniques, and sophisticated data science tools like machine learning has transformed this phase from a bottleneck into a catalyst for discovery. As these technologies continue to evolve, they will further accelerate the pace of rational design in metabolic engineering, enabling the more efficient development of robust microbial cell factories and improved crops to meet global challenges in health, energy, and food security.
The Design-Build-Test-Learn (DBTL) cycle is a foundational framework in metabolic engineering for the iterative development of microbial cell factories [1] [3]. In this cycle, the Learn phase serves as the critical bridge that transforms raw experimental data into actionable knowledge, informing and optimizing the designs for subsequent iterations. It is the engine of learning that converts the outcomes of the Test phase into hypotheses for a new Design phase. Without a robust Learn phase, DBTL cycles risk becoming merely empirical, time-consuming, and costly endeavors with diminished returns. Effective learning integrates both statistical analysis and model-guided assessment to decipher complex biological data, identify key limiting factors, and propose targeted genetic or process modifications [3] [42]. This article delves into the methodologies and tools that empower researchers to navigate the Learn phase, enabling a transition from simple data collection to profound biological insight and predictive engineering.
Analytical Methodologies in the Learn Phase
The Learn phase employs a dual-pronged analytical approach, leveraging both data-driven and mechanistic models to extract knowledge from experimental results.
Statistical and Machine Learning Analysis
Machine learning (ML) has emerged as a powerful tool for learning from data and proposing new designs when the relationship between genetic modifications and phenotypic outcomes is complex and not fully understood a priori [1].
- Application: ML models learn from a small set of experimentally probed strain designs (e.g., varying enzyme levels via promoter or RBS libraries) to predict the performance of untested designs and recommend the most promising ones for the next DBTL cycle [1].
- Algorithm Selection: In the low-data regime typical of early DBTL cycles, studies have shown that gradient boosting and random forest models often outperform other methods. These models are also demonstrated to be robust to common experimental challenges such as training set biases and measurement noise [1].
- Automated Recommendation: An algorithm can use ML model predictions to create a predictive distribution. This distribution is then sampled, based on a user-defined exploration/exploitation parameter, to recommend a new set of strains to build and test, thereby automating the iterative engineering process [1].
Model-Guided and Kinetic Analysis
In contrast to purely data-driven methods, mechanistic models are based on biological principles and provide deep insights into the underlying system dynamics.
- Kinetic Modeling: Kinetic models use ordinary differential equations (ODEs) to describe changes in intracellular metabolite concentrations over time. Each reaction flux is described by a kinetic mechanism derived from laws of mass action, making the parameters biologically interpretable [1] [42]. This allows for in silico perturbation of pathway elements, such as enzyme concentrations, to predict their effect on metabolic flux and identify bottlenecks [1].
- Framework for Benchmarking: Due to the scarcity and cost of generating multi-cycle public datasets, mechanistic kinetic models provide a valuable framework for benchmarking ML methods and optimizing DBTL cycle strategies in silico before committing to wet-lab experiments [1].
- Use Case: A kinetic model of a synthetic pathway in E. coli revealed non-intuitive behaviors; for instance, increasing the concentration of an individual enzyme sometimes led to a decrease in product flux due to substrate depletion, highlighting the necessity of combinatorial optimization guided by model insights [1].
Table 1: Comparison of Analytical Approaches in the Learn Phase.
| Feature | Statistical/Machine Learning Approach | Model-Guided/Kinetic Approach |
|---|---|---|
| Foundation | Data-driven correlations and patterns [1] | First principles and mechanistic biology [1] [42] |
| Data Requirements | Can be effective with limited data [1] | Requires kinetic parameters, often leading to large, underdetermined models [42] |
| Primary Output | Predictive models for strain performance [1] | Identification of rate-limiting steps and system dynamics [1] |
| Key Advantage | Handles complex, non-intuitive relationships without prior mechanistic knowledge [1] | Provides biological insight and is interpretable [1] [42] |
| Common Tools | Gradient Boosting, Random Forest [1] | Ordinary Differential Equation (ODE) models, Genome-Scale Models (GEMs) [1] [43] |
Implementation: A Workflow for the Learn Phase
Implementing an effective Learn phase requires a structured process to ensure that learning is systematic and actionable. The following workflow, derived from successful DBTL implementations, outlines the key steps.
Data Integration and Preprocessing
The first step involves aggregating heterogeneous data from the Test phase. This includes quantitative measurements of product titer, yield, rate (TYR), biomass, substrate consumption, and potentially metabolomics or proteomics data [1] [3]. This data must be cleaned, normalized, and integrated into a structured format suitable for analysis.
Hypothesis Generation and Model Selection
The integrated data is then analyzed to generate hypotheses about pathway limitations. The choice of analytical model depends on the research objective, the available data, and the experimental factors that can be manipulated [42]. The model must be able to represent these factors to produce actionable predictions.
Analysis and Knowledge Extraction
This is the core of the Learn phase, where the selected models are applied.
- For ML models, this involves training the model on the collected data and using it to predict the performance of a vast library of potential genetic designs (e.g., all possible promoter-gene combinations) [1].
- For kinetic models, this entails simulating the effect of perturbations (e.g., knocking down an enzyme like citrate synthase gltA to increase acetyl-CoA flux) and calculating new metabolic flux distributions [44].
Design Recommendation
The final output is a prioritized list of new strain designs for the next DBTL cycle. For ML, this could be a list of strains sampled from the predictive distribution [1]. For model-guided approaches, this is a set of genetic targets (e.g., genes to knockout or modulate) predicted to improve flux toward the desired product [44] [3].
A Case Study: Knowledge-Driven Learning for Dopamine Production
A 2025 study on optimizing dopamine production in E. coli provides a compelling example of a knowledge-driven Learn phase [3]. The researchers adopted a strategy that combined upstream in vitro investigation with in vivo DBTL cycling to accelerate learning.
- Initial Knowledge Gap: The first DBTL cycle often starts with limited prior knowledge, which can lead to suboptimal design choices and more iterations.
- Learn Phase Strategy: To address this, the team conducted in vitro tests using a crude cell lysate system to assess the functionality of the dopamine pathway and enzyme expression levels before moving to the more resource-intensive in vivo environment. This pre-DBTL learning provided crucial mechanistic insights and narrowed down the design space [3].
- Translation to In Vivo: The learning from the in vitro system was then translated to an in vivo context through high-throughput RBS engineering to fine-tune the expression levels of the key enzymes HpaBC and Ddc [3].
- Outcome: This knowledge-driven approach, which integrated learning from both in vitro and in vivo experiments, resulted in a dopamine production strain with a 2.6 to 6.6-fold improvement over the state-of-the-art, demonstrating the power of a robust and insightful Learn phase [3].
Table 2: Essential Research Reagents for Learn Phase Experiments.
| Reagent / Tool | Function in the Learn Phase |
|---|---|
| Kinetic Model (e.g., in SKiMpy) | Mechanistic simulation of metabolism to predict flux changes and identify bottlenecks [1]. |
| Machine Learning Algorithms (e.g., Random Forest) | Data-driven prediction of optimal strain designs from a large combinatorial space [1]. |
| RBS Library | A set of genetic parts for fine-tuning gene expression levels based on learned insights [3]. |
| Cell-Free Transcription-Translation System | In vitro testing of pathway functionality and enzyme kinetics to inform in vivo designs [3]. |
| Genome-Scale Model (GEM) | Constraint-based modeling to predict organism-wide metabolic capabilities and gene knockout targets [43] [42]. |
| Metabolomics & Fluxomics Datasets | Quantitative data on metabolite concentrations and metabolic fluxes for model validation and refinement [1] [42]. |
Strategic Considerations for Effective Learning
The setup of the DBTL cycle itself profoundly impacts the efficiency of the Learn phase. Strategic decisions can maximize the learning output from each experimental effort.
- Cycle Strategy: When the number of strains that can be built per cycle is limited, simulation studies suggest that starting with a larger initial DBTL cycle is more favorable than distributing the same total number of strains evenly across multiple cycles. A larger initial dataset provides a better foundation for machine learning models to make accurate predictions in subsequent cycles [1].
- Alignment of Model and Goal: The most critical step in model-guided learning is ensuring alignment between the research question, the available data, and the chosen modeling framework [42]. A model is only useful if it can represent the experimental inputs and produce predictions that are actionable for the specific engineering goal.
- Quantifiable Objectives: Success in the Learn phase must be measured against clear, pre-defined metrics. The primary metric is whether the learning leads to improved strain performance in the next cycle. Additionally, the predictive accuracy of models (ML or kinetic) when validated against new experimental data serves as a key performance indicator [1] [42].
The Learn phase is the intellectual core of the DBTL cycle, transforming metabolic engineering from a trial-and-error process into a predictive science. By strategically employing both statistical machine learning and mechanistic model-guided analysis, researchers can efficiently distill complex datasets into actionable knowledge. The continued development of computational tools, modeling frameworks, and high-throughput data generation will further enhance our ability to learn from each experiment. As these methodologies mature, the seamless integration of deep learning with kinetic models and the establishment of standardized, automated learning workflows promise to dramatically accelerate the rational design of efficient microbial cell factories for therapeutics and sustainable chemicals.
The Design-Build-Test-Learn (DBTL) cycle is an iterative framework central to modern metabolic engineering, enabling the systematic development of microbial cell factories for the production of valuable chemicals [45] [1] [46]. This process involves designing genetic modifications, building engineered strains, testing their performance, and learning from the data to inform the next design iteration. Isoprenoids, a vast class of natural products with applications in pharmaceuticals, fuels, and materials, represent a prime target for metabolic engineering due to their complex biosynthesis and commercial value [47] [45]. This case study examines the application of DBTL cycles to optimize the production of isoprenoids in Escherichia coli, focusing on the multivariate-modular engineering of the taxadiene pathway, which serves as a key intermediate for the anticancer drug Taxol [47]. We detail the experimental protocols, quantitative outcomes, and computational tools that have enabled remarkable improvements in isoprenoid titers, demonstrating how iterative DBTL cycles can overcome metabolic bottlenecks and achieve industrial-level production.
The DBTL Cycle in Metabolic Engineering
The DBTL cycle provides a structured approach for optimizing complex biological systems where rational design alone is insufficient due to limited knowledge of pathway regulation and complex cellular interactions [1]. In the Design phase, metabolic engineers identify target pathways, potential bottlenecks, and genetic elements for manipulation using computational models and prior knowledge. The Build phase involves the physical construction of engineered strains using synthetic biology tools, such as plasmid assembly, chromosome integration, and pathway refactoring. In the Test phase, the constructed strains are cultured under controlled conditions, and their performance is evaluated through analytics including titers, yields, productivity, and omics profiling. The Learn phase utilizes data analysis and modeling to extract insights from the experimental results, identify remaining limitations, and generate new hypotheses for the next design iteration [45] [1] [46]. This iterative process continues until the desired performance metrics are achieved.
Computational and Modeling Approaches
Kinetic modeling provides a mechanistic framework for simulating metabolic pathway behavior and predicting the effects of genetic perturbations before experimental implementation [1]. These models use ordinary differential equations to describe changes in metabolite concentrations over time, allowing researchers to simulate how variations in enzyme expression levels affect flux through the pathway. Machine learning algorithms, particularly gradient boosting and random forest models, have demonstrated strong performance in recommending optimal strain designs from limited experimental data, enabling more efficient navigation of the combinatorial design space [1]. These computational approaches are particularly valuable for identifying non-intuitive optimization strategies that might be missed through sequential engineering approaches.
Case Study: Taxadiene Production in E. coli
Pathway Design and Initial Engineering
Taxadiene serves as the first committed intermediate in the biosynthesis of Taxol, a potent anticancer drug originally isolated from the Pacific yew tree with significant production challenges [47]. The initial engineering strategy involved reconstructing the taxadiene biosynthetic pathway in E. coli by partitioning it into two modular units: the native upstream methylerythritol-phosphate (MEP) pathway that produces isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), and a heterologous downstream terpenoid-forming pathway converting these universal precursors to taxadiene [47]. This modular approach allowed for independent optimization of each pathway section, with the interface at IPP serving as a critical metabolic node.
Table 1: Key Enzymes in the Engineered Taxadiene Pathway
| Pathway Module | Enzyme | Gene | Source | Function |
|---|---|---|---|---|
| Upstream (MEP) | 1-deoxy-D-xylulose-5-phosphate synthase | dxs | E. coli | First committed step of MEP pathway |
| Upstream (MEP) | IPP isomerase | idi | E. coli | Interconversion of IPP and DMAPP |
| Downstream (Heterologous) | Geranylgeranyl diphosphate synthase | GGPS | Heterologous | Condensation of IPP/DMAPP to GGPP |
| Downstream (Heterologous) | Taxadiene synthase | TS | Taxus brevifolia | Cyclization of GGPP to taxadiene |
Multivariate-Modular Pathway Engineering
The conventional rational engineering approach of sequentially modifying pathway genes implicitly assumes linear, additive effects, which often fails due to complex nonlinear interactions, metabolite toxicity, and hidden regulatory pathways [47]. To address these limitations, researchers implemented a multivariate-modular pathway engineering strategy, simultaneously varying the expression of multiple genes within and between the two pathway modules [47]. This approach involved:
- Pathway Partitioning: Dividing the taxadiene pathway into upstream (MEP) and downstream (heterologous) modules separated at the IPP intermediate.
- Combinatorial Library Design: Constructing a library of 16 initial strains with varying expression levels of four rate-limiting upstream genes (dxs, idi, ispD, ispF) and the two downstream genes (GGPS, TS) using different promoter strengths and gene copy numbers.
- Expression Balancing: Systematically searching for optimal expression balances that maximized taxadiene production while minimizing the accumulation of inhibitory metabolites like indole.
This strategy revealed a highly nonlinear taxadiene flux landscape with a distinct global maximum, demonstrating that dramatic changes in production could be achieved within a narrow window of expression levels for the upstream and downstream pathways [47].
Experimental Protocols
Strain Construction and Pathway Assembly
Protocol 1: Modular Pathway Assembly
- Vector System Selection: Utilize a dual-plasmid system with compatible origins of replication and selective markers (e.g., pBb series with different copy numbers) [47] [48].
- Upstream Module Cloning: Clone the four key MEP pathway genes (dxs, idi, ispD, ispF) under the control of inducible promoters (e.g., Trc) into a medium-copy-number plasmid. Assemble as a synthetic operon with optimized ribosomal binding sites.
- Downstream Module Cloning: Clone the heterologous taxadiene synthesis genes (GGPS and TS) under control of strong inducible promoters (e.g., T7) into a high-copy-number plasmid. Test different gene orders (GGPS-TS vs TS-GGPS) as this can significantly impact titer.
- Chromosomal Integration: For reduced metabolic burden, integrate the upstream module operon into the E. coli chromosome using sites such as slr0168 or attB [47].
- Strain Transformation: Co-transform both plasmids into an appropriate E. coli host strain (e.g., DH1 or other K-12 derivatives with high transformation efficiency).
Fermentation and Analytics
Protocol 2: Fed-Batch Fermentation for Taxadiene Production
- Seed Culture Preparation: Inoculate single colonies into LB medium with appropriate antibiotics and grow overnight at 30-37°C with shaking.
- Bioreactor Inoculation: Transfer seed culture to a bioreactor containing defined minimal medium (e.g., M9 with glucose) with antibiotics to achieve initial OD600 of 0.05-0.1.
- Fermentation Conditions: Maintain temperature at 30°C, pH at 6.8-7.2, and dissolved oxygen above 30% saturation through aeration and agitation control.
- Induction Strategy: Add pathway inducer (e.g., IPTG for Trc promoter) during mid-exponential phase (OD600 ~0.6-0.8).
- Fed-Batch Operation: Once initial carbon source is depleted, initiate feeding with concentrated glucose solution (400-500 g/L) at exponential or constant rate to maintain metabolic activity while minimizing acetate formation.
- Product Extraction: Use an organic overlay (e.g., oleyl alcohol or dodecane) for in situ extraction of taxadiene to reduce product inhibition and degradation [48].
- Analytical Methods:
- Taxadiene Quantification: Analyze samples by GC-MS or LC-MS using external calibration curves with authentic standards.
- Metabolite Profiling: Quantify pathway intermediates (IPP, DMAPP, etc.) using LC-MS/MS with selected reaction monitoring.
- Protein Quantification: Determine enzyme expression levels via targeted proteomics (SRM) or Western blot.
Optimization Outcomes and Learnings
The multivariate-modular approach resulted in extraordinary improvements in taxadiene production. The optimized strain produced approximately 1.02 ± 0.08 g/L taxadiene in fed-batch bioreactor fermentations, representing a 15,000-fold increase over the control strain expressing only the native MEP pathway [47]. Key learnings from this iterative optimization included:
- Metabolic Burden Management: Strains with chromosomally integrated upstream pathways showed significantly higher production than plasmid-based systems, highlighting the importance of reducing metabolic burden.
- Nonlinear Pathway Interactions: The relationship between pathway expression strength and product titer was highly nonlinear, with sharp maxima that would be difficult to identify through sequential optimization.
- Inhibitory Metabolite Identification: The systematic approach revealed indole as an unexpected inhibitor of isoprenoid pathway activity, which could be mitigated through balanced pathway expression.
- Downstream Pathway Engineering: Subsequent engineering of P450-mediated oxidation successfully converted taxadiene to taxadien-5α-ol, demonstrating the extensibility of the optimized platform to later steps in Taxol biosynthesis [47].
Table 2: Quantitative Outcomes of Taxadiene Pathway Optimization
| Strain/Strategy | Taxadiene Titer | Fold Improvement | Key Innovation |
|---|---|---|---|
| Baseline (Native MEP only) | <0.1 mg/L | 1x | Native pathway |
| Initial Heterologous Pathway | ~10 mg/L | ~100x | Basic pathway expression |
| Modular Optimization | 1.02 ± 0.08 g/L | ~15,000x | Multivariate-modular balancing |
| P450 Oxidation Extension | 2,400x over yeast | N/A | Pathway expansion to taxadien-5α-ol |
Advanced Optimization Strategies
CRISPRi for Metabolic Flux Tuning
CRISPR interference (CRISPRi) has emerged as a powerful tool for fine-tuning metabolic pathways without permanent genetic modifications. This approach utilizes a catalytically dead Cas9 (dCas9) protein and guide RNAs (gRNAs) to repress transcription of target genes, enabling multiplexed downregulation of competing pathways [49]. In isoprenol production, researchers targeted 32 essential and non-essential genes in E. coli strains expressing either the mevalonate pathway or IPP-bypass pathway. The optimal CRISPRi strain achieved 12.4 ± 1.3 g/L isoprenol in 2-L fed-batch cultivation, demonstrating the scalability of this approach [49].
Protocol 3: CRISPRi Implementation for Pathway Optimization
- CRISPRi System Assembly: Clone dCas9 under control of an inducible promoter (e.g., Ptet) and array of gRNAs targeting selected genes under constitutive promoters.
- gRNA Design: Design 30-bp gRNAs targeting non-template DNA strands near start codons or promoter regions of genes to be repressed.
- Library Construction: Create a multiplexed gRNA library based on single guide RNA performance, focusing on genes whose repression improves product titer.
- Screening: Transform the CRISPRi system into production strains and screen for improved titers in multi-well plates before scale-up.
- Fed-Batch Validation: Scale promising strains to bioreactor cultivation to demonstrate industrial relevance.
Cofactor Engineering and Enzyme Optimization
Cofactor specificity represents another critical dimension for pathway optimization. In lactic acid production using cyanobacteria, researchers engineered lactate dehydrogenase (LDH) to preferentially utilize NADPH over NADH through site-directed mutagenesis, resulting in significantly improved productivity [50]. Similarly, in isoprenoid production, modifying the Shine-Dalgarno sequence of the phosphatase gene nudB increased its protein expression by 9-fold and reduced toxic IPP accumulation by 4-fold, leading to a 60% increase in 3-methyl-3-buten-1-ol yield [48].
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagent Solutions for Isoprenoid Pathway Engineering
| Reagent Category | Specific Examples | Function/Application |
|---|---|---|
| Vector Systems | pBb series, pTrc99A, pET vectors | Tunable expression of pathway genes with different copy numbers and promoter strengths |
| Promoter Systems | Trc, T7, lacUV5, Ptet | Controlled gene expression with varying induction mechanisms and strengths |
| Enzyme Variants | Archaeal mevalonate kinases, NudB phosphatases, P450 oxidases | Alternative enzymes with improved kinetics, specificity, or stability [48] [51] |
| CRISPR Tools | dCas9, gRNA scaffolds, aTc-inducible systems | Multiplexed gene repression for metabolic flux tuning [49] |
| Analytical Standards | Taxadiene, IPP, DMAPP, isoprenol | Quantification of pathway intermediates and products |
| Fermentation Additives | Oleyl alcohol overlay, dodecane | In situ product extraction to mitigate toxicity and inhibition |
| Host Strains | E. coli DH1, BL21, JM109 | Production hosts with varying metabolic backgrounds and transformation efficiencies |
The optimization of isoprenoid production in E. coli through iterative DBTL cycles demonstrates the power of systematic metabolic engineering approaches. The multivariate-modular strategy achieved remarkable 15,000-fold improvements in taxadiene production by balancing pathway expression and minimizing metabolic burden [47]. Emerging tools like CRISPRi further enable precise flux control, allowing researchers to simultaneously tune multiple pathway nodes [49]. The integration of kinetic modeling and machine learning promises to accelerate future DBTL cycles by better predicting optimal pathway configurations from limited experimental data [1]. As these technologies mature, the DBTL framework will continue to drive advances in microbial production of not only isoprenoids but a wide range of valuable natural products, strengthening the foundation for sustainable biomanufacturing.
The Design-Build-Test-Learn (DBTL) cycle is a foundational engineering framework in synthetic biology and metabolic engineering, enabling the systematic development of microbial cell factories [52]. This iterative process guides the transformation of a microorganism, such as E. coli, to efficiently produce target compounds, from initial design to performance optimization [53]. In metabolic engineering, the DBTL cycle's power lies in its structured approach to tackling biological complexity. Each iteration refines the metabolic system, progressively increasing the production yield of desired molecules like dopamine, a crucial neurotransmitter with significant pharmaceutical relevance [54] [52]. The integration of advanced computational tools and automation into the DBTL framework is shifting metabolic engineering from a traditionally artisanal, trial-and-error discipline toward a more predictable and efficient engineering science [54].
This case study examines the application of a knowledge-driven DBTL cycle for engineering an E. coli strain to produce dopamine. We focus on integrating modern tools, including artificial intelligence (AI) and machine learning (ML), with core biological principles to enhance the efficiency and success rate of strain development.
Design Phase: Rational Engineering of the Dopamine Pathway
The Design phase establishes the genetic blueprint for dopamine production in E. coli. This involves selecting a biosynthetic pathway, choosing appropriate genetic parts, and using computational models to predict the most effective engineering strategy.
Pathway and Enzyme Selection
Dopamine biosynthesis in engineered E. coli typically utilizes the L-tyrosine pathway. The key enzymatic steps involve converting the endogenous precursor L-tyrosine to L-DOPA by a tyrosine hydroxylase, followed by decarboxylation to dopamine by a DOPA decarboxylase.
Computational Strain Design with ET-OptME
A significant challenge in traditional algorithms is their reliance on stoichiometric models, which ignore enzymatic resource costs and reaction thermodynamics [55]. For this case study, we employ the ET-OptME framework, a novel algorithm that synergistically incorporates Enzyme constraints and Thermodynamic constraints into metabolic model simulations [55].
- Core Mechanism: ET-OptME consists of two complementary algorithms: ET-EComp, which identifies enzymes requiring up- or down-regulation by comparing their concentration ranges under different metabolic states, and ET-ESEOF, which scans for enzyme concentration trends as the target flux (dopamine production) is increased [55].
- Performance Advantage: As demonstrated in engineering Corynebacterium glutamicum, ET-OptME achieved a minimum 292% increase in precision and a 106% increase in accuracy over traditional stoichiometric algorithms like OptForce, while also outperforming other advanced enzyme-centric models [55]. This drastically improves the physiological relevance and experimental feasibility of predicted targets.
AI-Enhanced Predictive Design
Beyond constraint-based modeling, machine learning models can be trained on historical omics data and enzyme kinetics to predict optimal expression levels for pathway genes and identify potential hidden bottlenecks.
Table: Key Computational Tools for the Design Phase
| Tool Name | Type | Primary Function in Dopamine Project |
|---|---|---|
| ET-OptME | Metabolic Model Algorithm | Predicts high-precision, physiologically feasible gene knockout and regulation targets [55]. |
| Cameo | Software Platform | Performs strain simulation and optimization using various metabolic models [52]. |
| ECNet | Deep Learning Framework | Integrates evolutionary information to predict protein (enzyme) performance, useful for selecting optimal hydroxylase and decarboxylase variants [54]. |
| RetroPath 2.0 | Software Tool | Aids in designing metabolic pathways from available substrates [52]. |
The output of this phase is a prioritized list of genetic modifications: (1) introduction of heterologous genes for tyrosine hydroxylase (tyrH) and DOPA decarboxylase (ddc), and (2) targeted knockouts or down-regulations (e.g., pykA, pykF) and up-regulations (e.g., aroG, tyrA) in the central metabolism as predicted by ET-OptME to channel carbon flux toward L-tyrosine and dopamine.
Build Phase: High-Throughput Genetic Assembly
The Build phase translates the in silico design into physical DNA constructs and engineered living cells.
Automated DNA Assembly
Automation is critical for high-throughput and reproducible strain construction.
- Platforms: Biofoundries employ integrated robotic systems, such as the Opentrons liquid handling robot, which can be programmed via platforms like j5 or AssemblyTron for automated, high-fidelity DNA assembly [52].
- Method: Golden Gate assembly or Gibson Assembly is used to construct expression cassettes for the tyrH and ddc genes, along with the regulatory elements (promoters, RBSs) identified in the Design phase. These cassettes are then integrated into the E. coli chromosome at specified loci or placed on plasmids.
Multi-Parallel Strain Construction
A key advantage of automated biofoundries is the ability to build a library of variant strains in parallel. This library may include:
- Strains with different combinations of promoter strengths for the heterologous genes.
- Strains with the predicted gene knockouts performed in different sequences.
Quality Control
Constructed strains are validated using automated colony PCR and sequencing. Techniques like the Sequeduct pipeline, which uses Nanopore long-read sequencing, can verify the fidelity of large DNA constructs efficiently [52].
Table: Essential Research Reagents and Solutions for the Build Phase
| Reagent/Solution | Function | Example/Note |
|---|---|---|
| DNA Assembly Master Mix | Enzymatic assembly of DNA fragments. | Gibson Assembly Master Mix. |
| Automated Liquid Handler | Precise, high-throughput liquid transfer for setting up reactions. | Opentrons system [52]. |
| j5/AssemblyTron Software | Automates the design of DNA assembly protocols. | Ensures standardized, error-free instructions for robots [52]. |
| PCR Reagents & Oligos | Amplification of DNA parts and verification of constructs. | High-fidelity DNA polymerase. |
| Electrocompetent E. coli Cells | For transformation of assembled DNA. | High-efficiency strains like BW25113. |
| Selection Agar Plates | Growth medium for selecting successful transformants. | LB Agar with appropriate antibiotic (e.g., Kanamycin). |
Test Phase: Analytical Characterization of Strains
The Test phase involves culturing the built strain variants and quantitatively measuring their performance—specifically dopamine production and host cell fitness.
High-Throughput Fermentation
Strains are cultured in deep-well plates with controlled temperature and shaking. Automated systems can inoculate and monitor hundreds of cultures in parallel.
Analytical Chemistry for Metabolite Quantification
- Sample Preparation: Automated liquid handlers transfer culture broth at specific time points, remove cells via centrifugation or filtration, and prepare supernatants for analysis.
- Analysis Technique: Liquid Chromatography-Mass Spectrometry (LC-MS/MS) is the gold standard for quantifying dopamine and key metabolites (e.g., glucose, L-tyrosine, L-DOPA) in the medium. It provides high sensitivity and specificity.
- Fitness Metrics: Optical density (OD600) is measured to track cell growth, a key indicator of metabolic burden.
Rapid Screening with Biosensors
For ultra-high-throughput screening, LDBT (Learn-Design-Build-Test) approaches can be employed. This involves using machine learning models to guide the design of a strain library, which is then rapidly tested in cell-free systems [56]. Cell-free protein expression systems containing transcription/translation machinery can produce the dopamine pathway enzymes and report on their function in hours instead of days, providing a fast proxy for performance before moving to live-cell fermentation [56].
Learn Phase: Data Analysis and Model Refinement
The Learn phase is where data is transformed into knowledge, closing the DBTL loop. The performance data from the Test phase is analyzed to uncover the root causes of success or failure and to generate improved designs for the next cycle.
Data Integration and Multi-Omics Analysis
Data on metabolite concentrations, growth rates, and genetic constructs are aggregated. For deep learning, multi-omics analysis (transcriptomics, proteomics) can be performed on the best-performing strains to identify unexpected regulatory responses or metabolic bottlenecks not captured by the initial model [54].
Machine Learning for Pattern Recognition and Optimization
Machine learning algorithms are trained on the combined dataset (strain genotypes and phenotypes) to build predictive models.
- Algorithms: Gaussian Process Regression (GPR) is valuable as it provides predictions with uncertainty estimates, guiding the exploration of the design space. XGBoost and Artificial Neural Networks (ANNs) are also powerful tools for finding complex, non-linear relationships between genetic modifications and dopamine yield [54] [56].
- Function: The model might reveal, for instance, that a specific medium-range expression level of tyrH is optimal, or that an unanticipated gene (gene X) is highly correlated with high yield.
Hypothesis Generation for the Next DBTL Cycle
The insights gained lead to new, testable hypotheses. The output of the Learn phase is a refined strain design for the next Design phase, potentially including:
- Fine-tuning the expression of key genes based on ML-predicted optimal levels.
- Introducing new genetic modifications (e.g., knockdown of gene X) to alleviate a newly discovered bottleneck.
- Exploring a different set of enzyme variants for the pathway.
Table: Example Quantitative Data from an Iterative DBTL Cycle for Dopamine Production
| DBTL Cycle | Key Genetic Modifications | Max Dopamine Titer (mg/L) | Relative Increase | Key Learning |
|---|---|---|---|---|
| Cycle 1 (Base) | Introduction of tyrH and ddc genes. | 50 | Baseline | Base pathway functions but has low flux. |
| Cycle 2 | ET-OptME predicted knockouts (pykA, pykF); strong promoter on aroG. | 120 | 140% | Central metabolism redirection successful. L-tyrosine bottleneck identified. |
| Cycle 3 | ML-guided RBS library for tyrH; proteomics revealed burden. | 255 | 112% | Intermediate enzyme balance is more critical than maximal expression. |
| Cycle 4 | Incorporation of a more stable DOPA decarboxylase variant; knockdown of a competing pathway. | 450 | 76% | Enzyme stability and side-pathways limit final yield. |
This case study demonstrates that applying a knowledge-driven DBTL cycle, powered by advanced computational tools like the ET-OptME algorithm and machine learning, is a highly effective strategy for developing microbial cell factories for dopamine production [55]. The iterative process of designing, building, testing, and learning systematically uncovers and resolves complex metabolic bottlenecks that are impossible to predict a priori.
The future of DBTL cycles in metabolic engineering lies in increased autonomy and integration. Emerging trends include:
- AI-Powered Autonomous Biofoundries: Platforms like BioAutomata and AutoBioTech are demonstrating the feasibility of fully closed-loop DBTL cycles, where AI systems design experiments and robotic platforms execute them with minimal human intervention, dramatically accelerating project timelines [54] [52].
- Advanced Modeling and Biological Foundation Models: The development of Biological Large Language Models (BLMs) that can integrate information from DNA sequence to system-level physiology promises to make the Design phase even more predictive [54].
- Democratization via Cloud Labs: The coupling of AI design tools with remote-operated cloud laboratories will make this powerful DBTL approach accessible to a broader range of researchers and institutions, further accelerating innovation in metabolic engineering for biotechnology and drug development [54].
Overcoming DBTL Bottlenecks: From Cycle Involution to AI-Powered Optimization
The Design-Build-Test-Learn (DBTL) cycle represents a foundational framework in metabolic engineering and synthetic biology for systematically developing and optimizing biological systems [12]. This iterative process aims to engineer microorganisms for specific functions, such as producing valuable compounds including biofuels, pharmaceuticals, and fine chemicals [12] [9]. However, despite its structured approach, many research and development efforts encounter a significant challenge: the involution into endless, inefficient trial-and-error cycles that consume substantial time and resources without delivering proportional improvements.
This involution often stems from fundamental pitfalls in implementing the DBTL framework, particularly in the critical "Learn" phase where data should transform into actionable knowledge for subsequent cycles. When learning is inadequate, the cycle continues with minimal directional guidance, leading to random or suboptimal exploration of the vast biological design space. This technical analysis examines the common pitfalls perpetuating these inefficient cycles and presents validated methodologies to overcome them, leveraging recent advances in computational modeling, machine learning, and automated workflows.
Core Pitfalls in DBTL Implementation
Data Scarcity and the Learning Bottleneck
The effectiveness of any DBTL cycle hinges on the quality and quantity of data available for learning, yet this remains a critical bottleneck in many metabolic engineering projects. The fundamental challenge lies in the high-dimensional design space—encompassing promoters, ribosomal binding sites, gene sequences, and regulatory elements—that must be explored with limited experimental capacity [1]. Due to the costly and time-consuming nature of experiments, publicly available datasets encompassing multiple DBTL cycles are scarce, complicating systematic validation and comparison of machine learning methods and DBTL strategies [1].
Table 1: Impact of Initial Library Size on DBTL Cycle Efficiency
| Initial Library Size | Number of DBTL Cycles Needed | Resource Utilization | Success Rate |
|---|---|---|---|
| Small (≤ 16 variants) | High (> 4 cycles) | Inefficient | Low |
| Medium (~50 variants) | Moderate (3-4 cycles) | Balanced | Medium |
| Large (≥ 100 variants) | Low (1-2 cycles) | High initial investment | High |
Data from simulated DBTL cycles demonstrates that when the number of strains to be built is limited, starting with a large initial DBTL cycle is favorable over building the same number of strains for every cycle [1]. This approach provides sufficient initial data for machine learning models to identify meaningful patterns and make accurate predictions for subsequent cycles.
Inadequate Integration of Learning into Design
A second critical pitfall involves the failure to effectively translate learning from one cycle into improved designs for the next. Many DBTL implementations treat each cycle as largely independent rather than building cumulative knowledge. This disconnect often results from insufficient statistical analysis and inadequate modeling of complex pathway behaviors [9]. For instance, in combinatorial pathway optimization, simultaneous optimization of multiple pathway genes frequently leads to combinatorial explosions, making exhaustive experimental testing infeasible [1]. Without proper learning mechanisms, researchers default to intuitive rather than data-driven decisions.
The kinetic properties of metabolic pathways further complicate this challenge. Studies have shown that increasing enzyme concentrations of individual reactions does not always lead to higher fluxes but can instead decrease flux due to depletion of reaction substrates [1]. These non-intuitive dynamics underscore the necessity of computational models that can capture complex pathway behaviors and inform rational design strategies.
Suboptimal Experimental Design and Resource Allocation
Many DBTL cycles suffer from inefficient experimental designs that fail to maximize information gain per experimental effort. Traditional approaches often vary one factor at a time or use randomized selection of engineering targets, leading to more iterations and extensive consumption of time, money, and resources [3]. Additionally, the test phase frequently remains the throughput bottleneck in DBTL cycles, despite advances in other areas [57]. Without strategic experimental design, learning potential remains limited even with substantial experimental investment.
Computational and Modeling Solutions
Kinetic Modeling Frameworks for Predictive Simulation
Mechanistic kinetic models provide a powerful solution for simulating metabolic pathway behavior and predicting optimal engineering strategies. These models use ordinary differential equations to describe changes in intracellular metabolite concentrations over time, with each reaction flux described by a kinetic mechanism derived from mass action principles [1]. This approach allows for in silico changes to pathway elements, such as modifying enzyme concentrations or catalytic properties, enabling researchers to explore design spaces computationally before experimental implementation.
Table 2: Comparison of Metabolic Modeling Approaches in DBTL Cycles
| Model Type | Key Features | Best Use Cases | Limitations |
|---|---|---|---|
| Kinetic Models | Captures dynamic metabolite concentrations; describes reaction fluxes via ODEs | Pathway optimization; understanding metabolic dynamics | Requires extensive parameterization; computationally intensive |
| Flux Balance Analysis (FBA) | Constraint-based; predicts flux distributions at steady state | Genome-scale predictions; growth-coupled production | Limited dynamic information; depends on objective function selection |
| Thermodynamics-Based FBA | Incorporates thermodynamic constraints on reaction fluxes | Assessing pathway feasibility; energy balance analysis | Increased complexity; requires thermodynamic parameters |
| Pareto Optimal Engineering | Multi-objective optimization balancing competing goals | Identifying trade-offs between growth and production | Complex implementation; solution selection challenges |
The application of these modeling frameworks shows significant promise in reducing experimental cycles. For instance, Pareto optimal metabolic engineering has successfully identified gene knockout strategies in S. cerevisiae that balance multiple objectives including growth rate, production capability, and genetic modification complexity [58].
Machine Learning for Pattern Recognition and Prediction
Machine learning methods offer powerful tools for learning from experimental data and proposing new designs for subsequent DBTL cycles. In the low-data regime typical of early DBTL cycles, gradient boosting and random forest models have demonstrated robust performance, showing resilience to training set biases and experimental noise [1]. These methods can identify complex, non-linear relationships between genetic modifications and metabolic outcomes that might escape conventional statistical analysis.
Advanced implementations now incorporate deep learning approaches trained on single-cell level metabolomics data. The RespectM method, for example, can detect metabolites at a rate of 500 cells per hour with high efficiency, generating thousands of single-cell metabolomics data points that represent metabolic heterogeneity [59]. This "heterogeneity-powered learning" approach trains optimizable deep neural networks to suggest minimal operations for achieving high production targets, such as triglyceride production [59].
Experimental Protocols for Efficient DBTL Cycling
Knowledge-Driven DBTL with Upstream In Vitro Investigation
A knowledge-driven DBTL cycle incorporating upstream in vitro investigation provides a robust methodology for accelerating strain development while generating mechanistic insights [3]. This approach was successfully implemented for optimizing dopamine production in E. coli, achieving a 2.6 to 6.6-fold improvement over state-of-the-art production methods.
Diagram 1: Knowledge-driven DBTL workflow with upstream in vitro investigation
Protocol: Knowledge-Driven DBTL for Metabolic Pathway Optimization
Upstream In Vitro Investigation Phase
- Cell Lysate Preparation: Prepare crude cell lysate systems from production host (e.g., E. coli FUS4.T2) to maintain metabolite pools and energy equivalents [3].
- Reaction Buffer Setup: Prepare phosphate buffer (50 mM, pH 7) supplemented with 0.2 mM FeCl₂, 50 μM vitamin B₆, and pathway substrates (1 mM l-tyrosine or 5 mM l-DOPA for dopamine production) [3].
- Enzyme Expression Testing: Test different relative expression levels of pathway enzymes in cell-free systems to identify optimal ratios before in vivo implementation.
In Vivo Implementation Phase
- RBS Library Construction: Design and build ribosome binding site (RBS) libraries focusing on Shine-Dalgarno sequence modulation without interfering secondary structures [3].
- High-Throughput Screening: Implement automated 96-well plate cultivation with appropriate media (e.g., minimal medium with 20 g/L glucose, 10% 2xTY, and necessary supplements) [3].
- Analytical Methods: Employ fast ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) for quantitative analysis of target products and key intermediates [9].
Automated DBTL Pipeline Implementation
Fully automated DBTL pipelines represent the state-of-the-art in overcoming iterative inefficiencies. These integrated systems combine computational design tools with robotic assembly and high-throughput analytics to dramatically accelerate cycle turnover [9].
Protocol: Automated DBTL Pipeline for Pathway Optimization
Design Stage
- Pathway Design: Use RetroPath and Selenzyme tools for automated pathway and enzyme selection [9].
- Parts Design: Employ PartsGenie software for designing reusable DNA parts with optimized ribosome-binding sites and codon-optimized coding regions [9].
- Library Reduction: Apply design of experiments (DoE) based on orthogonal arrays combined with Latin square design to reduce combinatorial libraries to tractable sizes (e.g., 2592 to 16 constructs) while maintaining statistical representativeness [9].
Build Stage
- Automated Assembly: Implement ligase cycling reaction (LCR) on robotics platforms following automated worklist generation [9].
- Quality Control: Perform high-throughput automated plasmid purification, restriction digest, and analysis by capillary electrophoresis, followed by sequence verification.
Test Stage
- Cultivation: Execute automated 96-deepwell plate growth and induction protocols with optimized media and conditions.
- Analytics: Employ quantitative UPLC-MS/MS with high mass resolution for target product detection.
- Data Processing: Utilize custom R scripts for automated data extraction and processing.
Learn Stage
Diagram 2: Automated DBTL pipeline with integrated biofoundry approaches
The Scientist's Toolkit: Essential Research Reagent Solutions
Table 3: Key Research Reagents and Their Applications in DBTL Cycles
| Reagent/Resource | Function | Application Example | Considerations |
|---|---|---|---|
| Ribosome Binding Site (RBS) Libraries | Fine-tuning translation initiation rates | Optimizing relative enzyme expression in metabolic pathways | SD sequence modulation preserves secondary structure |
| Cell-Free Protein Synthesis (CFPS) Systems | Rapid enzyme testing bypassing cellular constraints | Pre-optimizing pathway enzyme ratios before in vivo work | Crude cell lysate maintains metabolite pools |
| Specialized Minimal Media | Controlled cultivation conditions | High-throughput screening of production strains | Precise supplementation prevents bottlenecks |
| Mass Spectrometry Standards | Quantitative metabolite analysis | Absolute quantification of pathway products and intermediates | Isotope-labeled internal standards for accuracy |
| Automated DNA Assembly Reagents | High-throughput construct generation | Building combinatorial pathway libraries | Ligase cycling reaction enables complex assemblies |
| Pathway-Specific Substrates | Feeding precursor molecules | l-tyrosine for dopamine production; malonyl-CoA for flavonoids | Cofactor balancing critical for efficiency |
Overcoming endless trial-and-error cycles in metabolic engineering requires systematic approaches that address the fundamental bottlenecks in DBTL implementation. The integration of computational modeling, machine learning, and automated workflows provides a robust framework for breaking free from inefficient iterations. Key strategies include:
- Invest in Comprehensive Initial Characterization: Utilize upstream in vitro investigations and kinetic modeling to generate foundational knowledge before full DBTL cycling.
- Implement Strategic Experimental Design: Apply DoE methodologies to maximize information gain from limited experimental resources.
- Leverage Machine Learning Capabilities: Employ appropriate algorithms (gradient boosting, random forest) that perform well in low-data regimes to extract maximal insights from limited datasets.
- Automate Where Possible: Implement automated biofoundry approaches to increase throughput, reduce human error, and accelerate cycle turnover.
By addressing these core areas, metabolic engineers can transform their DBTL cycles from endless trial-and-error loops into efficient, knowledge-driven processes that systematically converge on optimal solutions, ultimately accelerating the development of robust microbial cell factories for sustainable bioproduction.
The Role of Machine Learning in Resolving Non-Intuitive Metabolic Interactions
The Design-Build-Test-Learn (DBTL) cycle serves as the fundamental framework for modern metabolic engineering, providing a systematic process for developing microbial cell factories. This iterative cycle begins with the Design of genetic modifications, proceeds to the Build phase where these designs are implemented in a host organism, advances to the Test phase where performance is experimentally characterized, and culminates in the Learn phase where data analysis informs the next design iteration [60] [61]. However, a fundamental challenge has persistently hampered the efficiency of this process: our inability to accurately predict complex cellular behaviors after modifying genotypes, particularly non-intuitive metabolic interactions [62] [61].
These non-intuitive interactions—including allosteric regulation, post-translational modifications, and pathway channeling—create unpredictable dynamics in engineered biological systems [62] [63]. Traditional kinetic models struggle to capture these complexities because they require extensive domain expertise, significant development time, and rely on mechanistic assumptions about underlying relationships that are often incompletely characterized [62]. This knowledge gap forces metabolic engineers to rely on extensive empirical iteration rather than predictive engineering, dramatically increasing development time and resources [61].
Machine learning (ML) is now revolutionizing how we approach these challenges by transforming the DBTL cycle. By leveraging large biological datasets, ML models can detect complex patterns in high-dimensional spaces, enabling them to identify non-obvious relationships between genetic modifications and metabolic phenotypes [60] [61]. This capability is particularly valuable for predicting non-intuitive metabolic interactions that elude traditional modeling approaches. Recent advances have even prompted a re-evaluation of the traditional DBTL sequence, with some researchers proposing a restructured "LDBT" (Learn-Design-Build-Test) approach where machine learning precedes design, potentially enabling functional solutions in a single cycle [60].
Machine Learning Approaches for Decoding Metabolic Interactions
Supervised Learning for Predictive Modeling of Pathway Dynamics
Supervised machine learning provides a powerful alternative to traditional kinetic modeling for predicting metabolic pathway dynamics. This approach learns the function connecting metabolite and protein concentrations to reaction rates directly from experimental data, without presuming specific mechanistic relationships [62]. The mathematical foundation involves treating metabolic dynamics as a supervised learning problem where the function ( f ) in the system of ordinary differential equations ( \dot{m}(t) = f(m(t), p(t)) ) is approximated by machine learning algorithms. Here, ( \dot{m}(t) ) represents metabolite time derivatives, while ( m(t) ) and ( p(t) ) denote metabolite and protein concentration vectors, respectively [62].
The model is trained by solving an optimization problem that minimizes the difference between predicted and observed metabolite time derivatives across multiple time series datasets:
[ \arg \min{f} \sum{i=1}^{q} \sum{t \in T} \left\lVert f(\tilde{m}i[t], \tilde{p}i[t]) - \dot{\tilde{m}}i(t) \right\rVert^2 ]
where ( i ) represents different experimental strains (time series), and ( T ) represents observation time points [62]. This approach has demonstrated superior performance compared to classical Michaelis-Menten models, particularly for predicting dynamics in limonene and isopentenol biosynthetic pathways, even when trained on limited data (as few as two time series) [62].
The SCOUR Framework for Identifying Regulatory Interactions
For identifying specific metabolite-enzyme regulatory relationships, the Stepwise Classification of Unknown Regulation (SCOUR) framework provides a specialized machine learning approach. SCOUR addresses the critical challenge of limited training data for metabolic regulation through an "autogeneration" strategy that synthetically creates training data, enabling the application of established classification algorithms to identify regulatory interactions [63].
This framework employs a stepwise process that progressively identifies reactions controlled by one, two, or three metabolites. Each step uses different classification features and operates independently, though the stepwise approach significantly reduces the hypothesis space that must be explored. When applied to realistic conditions (low sampling frequency and high noise), SCOUR achieves high accuracy in identifying single-metabolite controllers, with predictive performance for two-metabolite controllers ranging from 32% to 88% positive predictive value (PPV) for noiseless data, and 6.6% to 27% PPV for high-noise, low-frequency data—still significantly better than random classification [63].
Protein Language Models for Enzyme Optimization
At the protein level, large language models (LLMs) originally developed for natural language processing have been adapted to address challenges in enzyme engineering. Models such as ESM-2 and EVmutation can predict the functional effects of protein sequence variations, enabling more efficient exploration of sequence space [2]. These models learn from evolutionary patterns captured in vast databases of protein sequences and structures, allowing them to identify non-obvious sequence modifications that optimize enzyme function [60].
Protein language models have demonstrated remarkable capability in zero-shot prediction—designing functional proteins without additional training—as shown in applications ranging from engineering TEV protease variants with improved catalytic activity to developing stabilized hydrolases for PET depolymerization [60]. When integrated into autonomous enzyme engineering platforms, these models have achieved substantial improvements, such as a 26-fold enhancement in phytase activity at neutral pH and a 16-fold improvement in ethyltransferase activity, accomplishing in four weeks what might otherwise require extensive experimental iteration [2].
Experimental Methodologies and Workflows
Data Requirements and Preparation for Metabolic ML
Successful application of machine learning to metabolic interaction analysis requires specific types and quality of experimental data. The following table outlines key data requirements and their applications in ML modeling:
Table 1: Data Requirements for Machine Learning in Metabolic Interaction Studies
| Data Type | Specific Applications | Key Considerations | Example ML Use |
|---|---|---|---|
| Time-series metabolomics | Dynamic pathway modeling, Flux prediction | Sampling frequency, Coverage of pathway intermediates | Supervised learning of metabolic dynamics [62] |
| Proteomics | Enzyme level quantification, Input for kinetic models | Correlation with actual enzyme activities | Feature in dynamic models [62] |
| Enzyme kinetics | Training data for stability/activity predictors | Standardized assay conditions | DeepSol for solubility; Prethermut for stability [60] |
| Fluxomics | Ground truth for reaction rates, Regulation identification | Integration with metabolite data | SCOUR framework for allosteric regulation [63] |
| Multi-omics integration | Holistic pathway analysis, Host effects prediction | Data alignment across modalities | iPROBE for pathway optimization [60] |
Protocol: ML-Guided Identification of Allosteric Regulators Using SCOUR
Objective: Identify potential allosteric regulators of a specific metabolic reaction using the SCOUR framework.
Step 1: Data Collection and Preprocessing
- Collect time-course measurements of intracellular metabolite concentrations (metabolomics) and metabolic fluxes (fluxomics) under multiple perturbation conditions [63].
- Preprocess data to handle missing values, normalize measurements, and calculate derivatives where needed.
- Generate synthetic training data through "autogeneration" strategy to overcome limited experimental examples [63].
Step 2: Feature Engineering
- For each reaction-metabolite pair, compute correlation metrics between metabolite concentrations and reaction fluxes.
- Calculate additional features including concentration-flux cross-correlations at different time lags and statistical moments of concentration distributions [63].
- Normalize features to standard distributions for model compatibility.
Step 3: Model Training and Validation
- Train ensemble classifiers (Random Forest, XGBoost) to distinguish regulating from non-regulating metabolite-reaction pairs [63].
- Implement stepwise classification: first identify single-metabolite controllers, then two-metabolite, then three-metabolite interactions.
- Validate model performance using cross-validation and holdout test sets, calculating precision-recall metrics focused on positive predictive value [63].
Step 4: Experimental Validation
- Prioritize top predictions based on model confidence scores for experimental testing.
- Design enzyme assays with predicted regulator metabolites to confirm allosteric effects [63].
- Iterate model with newly confirmed interactions to improve predictive performance.
Protocol: Supervised Learning for Pathway Dynamics Prediction
Objective: Develop a machine learning model to predict metabolic pathway dynamics from proteomics and metabolomics data.
Step 1: Training Data Generation
- Engineer multiple strain variants with varying expression levels of pathway enzymes.
- For each strain, collect dense time-series measurements of metabolite and protein concentrations throughout the cultivation period [62].
- Calculate metabolite time derivatives ( \dot{m}(t) ) from concentration measurements using numerical differentiation [62].
Step 2: Model Architecture Selection
- Select appropriate ML algorithms based on data characteristics: Gaussian process regressors for small datasets, neural networks for large multi-omics datasets [62] [61].
- Define input features (current metabolite and protein concentrations) and output variables (metabolite time derivatives).
Step 3: Model Training and Tuning
- Split data into training (70%), validation (15%), and test (15%) sets.
- Train model to minimize difference between predicted and actual metabolite time derivatives.
- Employ regularization techniques to prevent overfitting, particularly with limited training examples.
Step 4: Model Application
- Use trained model to predict dynamics of new strain designs in silico.
- Select most promising candidates for experimental implementation [62].
- Continuously update model with new experimental results to improve predictive accuracy.
Visualization of Workflows and Metabolic Relationships
The Machine Learning-Enhanced DBTL Cycle for Metabolic Engineering
The following diagram illustrates how machine learning transforms the traditional DBTL cycle, particularly through the emerging LDBT paradigm that begins with learning:
The SCOUR Framework for Identifying Metabolic Regulation
This diagram outlines the stepwise machine learning approach for identifying metabolite-enzyme regulatory interactions:
Performance Comparison of ML Approaches in Metabolic Engineering
Table 2: Performance Metrics of Machine Learning Methods for Metabolic Interaction Prediction
| ML Method | Application Scope | Key Performance Metrics | Data Requirements | Limitations |
|---|---|---|---|---|
| Supervised Learning for Pathway Dynamics [62] | Predicting metabolite dynamics in engineered pathways | Outperformed Michaelis-Menten models; Accurate prediction with only 2 time series | Time-series metabolomics & proteomics | Requires dense time-course data |
| SCOUR Framework [63] | Identifying allosteric regulatory interactions | PPV: 32-88% (noiseless data); 6.6-27% (noisy data) for 2-metabolite controllers | Metabolomics & fluxomics under multiple conditions | Performance decreases with interaction complexity |
| Protein Language Models (ESM-2) [2] | Enzyme engineering and optimization | 26-fold activity improvement in 4 weeks; 59.6% of variants above WT baseline | Protein sequence databases; Fitness data | Limited extrapolation beyond training distribution |
| Consensus Metabolite-DDI Models [64] | Predicting drug-drug interactions via CYP450 | Accuracy: 0.793-0.795; AUC: ~0.9 | Substrate/inhibitor datasets for CYP isozymes | Focused on pharmacokinetic interactions only |
| Cell-free + ML Screening [60] | High-throughput protein variant testing | Screening of >100,000 reactions; 10-fold increase in design success | Cell-free expression data; Deep sequencing | Specialized equipment requirements |
Essential Research Reagents and Computational Tools
Table 3: Research Reagent Solutions for ML-Driven Metabolic Engineering
| Reagent/Tool Category | Specific Examples | Function in Workflow | Key Features |
|---|---|---|---|
| ML Model Architectures | ESM-2, ProteinMPNN, EVmutation [60] [2] | Protein variant prediction and design | Zero-shot prediction; Evolutionary scale training |
| Specialized Enzymes | Halide methyltransferase (AtHMT), Phytase (YmPhytase) [2] | Model evaluation and validation | High-throughput assay compatibility |
| Cell-Free Expression Systems | PURE system, Crude cell lysates [60] [3] | Rapid protein production and testing | Bypass cellular constraints; Enable ultra-high-throughput screening |
| Metabolomics Platforms | LC-MS, GC-MS, NMR platforms | Generate training data for ML models | Quantitative concentration data; Broad metabolite coverage |
| Automated Biofoundries | iBioFAB, ExFAB [60] [2] | Integrated DBTL automation | End-to-end workflow integration; High reproducibility |
| Allosteric Regulation Predictors | AlloFinder [63] | Computational identification of regulatory sites | Structure-based prediction; Molecular docking |
Machine learning has fundamentally transformed our approach to resolving non-intuitive metabolic interactions within the DBTL cycle. By leveraging patterns in large biological datasets, ML models can identify complex relationships that escape traditional mechanistic modeling, enabling more predictive metabolic engineering and reducing reliance on costly experimental iteration. The integration of machine learning at multiple stages of the DBTL cycle—from initial protein design using language models to the identification of regulatory interactions with frameworks like SCOUR—has created new paradigms for biological engineering.
Looking forward, several emerging trends promise to further advance this field. The development of foundation models trained on massive biological datasets will enhance zero-shot prediction capabilities, potentially reducing the need for extensive training data specific to each engineering project [60]. The rise of autonomous experimentation platforms that fully integrate ML with biofoundry automation will accelerate the DBTL cycle, as demonstrated by systems that have engineered enzyme improvements of over 20-fold in just four weeks [2]. Finally, the creation of more sophisticated multi-scale models that integrate information from protein sequences to ecosystem dynamics will provide increasingly comprehensive understanding of metabolic interactions, ultimately enabling true design-based engineering of biological systems with minimal iterative optimization.
The Design-Build-Test-Learn (DBTL) cycle serves as a fundamental framework in synthetic biology and metabolic engineering for systematically developing and optimizing biological systems [65]. This iterative process enables researchers to engineer organisms for specific functions, such as producing biofuels or pharmaceuticals [12]. However, a significant bottleneck has emerged in the "Learn" phase, where researchers struggle to extract meaningful insights from complex biological data to inform the next design iteration [65]. This challenge becomes particularly acute in low-data regimes, where limited experimental data is available, a common scenario in early-stage metabolic engineering projects.
Machine learning (ML) promises to revolutionize the DBTL cycle by enabling data-driven predictions, but algorithm selection critically depends on performance in data-scarce environments [65]. This technical review benchmarks two prominent ensemble ML algorithms—Random Forest (RF) and Gradient Boosting Machines (GBM)—specifically for low-data scenarios within metabolic engineering. RF employs a bagging approach that builds multiple independent decision trees, while GBM utilizes a boosting technique that sequentially builds trees to correct previous errors [66]. Understanding their relative performance characteristics provides researchers with actionable guidance for implementing ML-driven learning in constrained data environments.
Algorithm Fundamentals and DBTL Integration
Core Algorithmic Principles
Random Forest operates on the principle of bootstrap aggregation (bagging), creating multiple decision trees from random subsets of the training data and features [66]. This independence between trees makes RF robust to overfitting, especially valuable with limited data. The final prediction typically averages individual tree outputs (for regression) or uses majority voting (for classification). RF's inherent randomness provides stability, and the algorithm naturally generates out-of-bag error estimates for performance validation without requiring a separate validation set—a significant advantage in low-data regimes [66].
Gradient Boosting Machines employ a fundamentally different boosting approach, building trees sequentially where each new tree corrects errors made by previous ones [66]. GBM optimizes a loss function using gradient descent, gradually reducing prediction bias. Unlike RF's parallel tree construction, GBM's sequential nature creates dependency between trees, potentially achieving higher accuracy but with increased risk of overfitting on small datasets. The algorithm requires careful hyperparameter tuning (learning rate, tree complexity, number of iterations) to generalize well [66].
Integration within the DBTL Cycle
The DBTL cycle provides a structured framework for metabolic engineering, where ML algorithms serve as computational engines in the "Learn" phase [65]. As illustrated in Figure 1, experimental data from "Test" phases feeds into ML models to generate predictive insights for subsequent "Design" iterations. This creates a virtuous cycle of data refinement where each DBTL iteration enhances dataset quality and model accuracy.
Figure 1: ML Integration in the DBTL Cycle
In metabolic engineering applications, ML algorithms can predict metabolic behaviors, optimize pathway designs, or identify key genetic modifications by learning from previous "Build" and "Test" cycles [67]. For instance, ML models can predict enzyme performance under specific conditions or identify promising pathway variants, significantly accelerating the DBTL cycle by reducing the experimental space that must be empirically tested [65].
Performance Benchmarking in Low-Data Regimes
Comparative Performance Analysis
A rigorous study directly compared RF and GBM performance on small datasets comprising categorical variables, highly relevant to metabolic engineering where strain characteristics and experimental conditions often represent categorical features [66]. The research established 690 building datasets through careful preprocessing and standardization, then evaluated algorithms using leave-one-out cross-validation (LOOCV)—particularly suitable for small datasets as it maximizes training data utilization [66].
As shown in Table 1, RF demonstrated superior stability and accuracy for most predictive tasks in data-scarce environments, though GBM achieved competitive performance in specific applications.
Table 1: Performance Benchmark of RF vs. GBM on Small Datasets [66]
| Performance Metric | Random Forest (RF) | Gradient Boosting (GBM) | Experimental Context |
|---|---|---|---|
| Overall Stability | Superior | Moderate | Small datasets (690 samples) with categorical variables |
| Average Accuracy | Higher | Lower | Prediction models for demolition waste generation |
| Specific Application Performance | Consistent across most models | Excellent in some specific models | Performance varied by waste type |
| Key Strengths | Stable predictions, robust to overfitting | Can achieve excellent performance in specific cases | |
| R² Values | >0.6 (most models) | >0.6 (most models) | Excellent performance threshold |
| R Values | >0.8 (most models) | >0.8 (most models) | Excellent performance threshold |
Further supporting evidence comes from aerospace engineering, where RF's Extremely Randomized Trees algorithm achieved the highest coefficient of determination (R²) for predicting airfoil self-noise, while GB variants offered advantages in training efficiency [68]. This cross-domain validation reinforces that RF's robustness extends beyond biological contexts.
Algorithm Selection Guidelines
Based on empirical evidence, researchers should consider the following guidelines for algorithm selection in low-data metabolic engineering applications:
Prioritize Random Forest when working with small datasets (<1000 samples) comprising mainly categorical variables [66]. RF's bagging approach provides more stable predictions and superior resistance to overfitting.
Consider Gradient Boosting when pursuing maximum predictive accuracy for specific well-defined tasks and when sufficient computational resources are available for extensive hyperparameter tuning [66] [68].
Employ LOOCV rather than k-fold cross-validation for model evaluation in low-data regimes, as it maximizes training data utilization and provides more reliable performance estimates [66].
Utilize RF's inherent feature importance metrics to identify key biological variables, which can inform subsequent DBTL cycles by highlighting the most influential genetic or environmental factors [66].
Experimental Protocols for Algorithm Implementation
Data Preprocessing and Feature Engineering
Metabolic engineering data requires specialized preprocessing to ensure ML model efficacy:
Handle Categorical Variables: Convert biological conditions (e.g., strain type, promoter strength, media composition) using one-hot encoding or target encoding to make them amenable to tree-based algorithms [66].
Eliminate Outliers: Identify and remove statistical outliers that may skew model training, particularly critical in small datasets where outliers exert disproportionate influence [66].
Normalize Numerical Features: Apply standardization (zero mean, unit variance) or normalization (scaling to [0,1] range) to ensure consistent feature scaling [66].
Address Data Imbalance: Employ stratification during cross-validation to maintain class distribution, crucial for biological datasets where certain metabolic outcomes may be rare [66].
Model Training and Validation Framework
Implementing a rigorous training protocol ensures reliable model performance:
Hyperparameter Tuning: Conduct systematic hyperparameter optimization using grid or random search. Critical parameters include:
- RF: number of trees, maximum depth, minimum samples split
- GBM: learning rate, number of boosting stages, subsampling ratio [66]
Validation Methodology: Apply LOOCV for datasets under 1000 samples [66]. For each iteration, use:
- Training set: n-1 samples
- Test set: 1 sample
- Repeat n times with different test samples
Performance Metrics: Employ multiple evaluation metrics to comprehensively assess model performance:
- R² (Coefficient of Determination): Measures proportion of variance explained
- RMSE (Root Mean Square Error): Quantifies absolute prediction error
- MAE (Mean Absolute Error): Provides interpretable error magnitude
- Pearson's R: Assesses prediction-actual value correlation [66]
Figure 2: LOOCV Workflow for Small Datasets
Metabolic Engineering Applications and Case Studies
Predictive Modeling for Metabolic Flux Optimization
Machine learning algorithms can predict metabolic behaviors by learning from previous DBTL cycles. RF has demonstrated particular utility for predicting metabolic flux distributions in engineered strains, enabling in silico testing of genetic modifications before laboratory implementation [67]. For instance, ML models can predict how knockout or amplification of specific enzymes affects product yield, guiding the design of subsequent strain engineering iterations.
The co-FSEOF (co-production using Flux Scanning based on Enforced Objective Flux) algorithm represents a specialized approach for identifying metabolic engineering targets to co-optimize multiple metabolites [69]. When integrated with RF or GBM, this enables prediction of intervention strategies for synergistic product formation, such as identifying reaction deletions/amplifications that simultaneously enhance production of both primary and secondary metabolites [69].
Research Reagent Solutions for ML-Driven Metabolic Engineering
Implementing ML-guided DBTL cycles requires specific experimental tools and reagents. Table 2 summarizes essential resources for generating high-quality data for ML models.
Table 2: Research Reagent Solutions for ML-Driven Metabolic Engineering
| Reagent/Resource | Function | Application in DBTL Cycle |
|---|---|---|
| Genome-Scale Metabolic Models (GEMs) | In silico representation of metabolic network | Predict metabolic fluxes and identify engineering targets [69] |
| Plasmid Systems (Dual-Plasmid) | Tunable gene expression control | Systematically optimize pathway expression levels [70] |
| Automated Strain Construction Tools | High-throughput genetic modification | Rapidly build diverse strain variants for training data [71] |
| Analytical Standards (LC-MS/MS) | Quantitative metabolite profiling | Generate accurate training data for ML models [67] |
| Fluorescent Reporter Proteins | Real-time monitoring of pathway activity | Provide dynamic data for ML-based pathway optimization [70] |
Future Perspectives and Implementation Challenges
Emerging Trends and Opportunities
The integration of ML into metabolic engineering DBTL cycles is accelerating through several key developments:
Automated Biofoundries: High-throughput automated facilities enable rapid construction and testing of thousands of genetic variants, generating the extensive datasets needed for robust ML model training [71]. These systems address the data scarcity challenge by massively parallelizing the "Build" and "Test" phases.
Multi-Omics Data Integration: Combining genomics, transcriptomics, proteomics, and metabolomics data provides comprehensive training inputs for ML models, enhancing their predictive accuracy for complex metabolic behaviors [67].
Explainable AI (XAI): Advanced ML techniques that provide interpretable predictions are particularly valuable for metabolic engineering, where understanding biological mechanisms remains crucial for rational design [65].
Implementation Challenges and Mitigation Strategies
Despite promising advances, significant challenges remain in applying ML to metabolic engineering:
Data Scarcity: Early-stage projects often lack sufficient data for robust ML training. Potential solutions include:
- Transfer learning from related organisms or pathways
- Data augmentation through synthetic data generation
- Strategic experimental design to maximize information gain per experiment
Biological Complexity: Cellular systems exhibit non-linear, context-dependent behaviors difficult to capture in ML models. Hybrid approaches combining mechanistic models with data-driven ML show promise for addressing this limitation [67].
Model Interpretability: While tree-based algorithms provide some feature importance metrics, extracting biologically meaningful insights remains challenging. Researchers should complement ML predictions with domain expertise and experimental validation.
Benchmarking analyses establish that Random Forest generally outperforms Gradient Boosting Machines in low-data regimes typical of early-stage metabolic engineering projects. RF's superior stability, robustness to overfitting, and reliable performance with categorical variables make it particularly suitable for the data-scarce environments often encountered in biological research [66]. However, GBM remains valuable for specific applications where maximum predictive accuracy is required and sufficient resources exist for extensive hyperparameter optimization.
Integrating these ML algorithms into the DBTL cycle addresses critical bottlenecks in the "Learn" phase, enabling data-driven insights that inform subsequent design iterations [65]. As synthetic biology continues evolving toward more predictive engineering, ML algorithms will play increasingly vital roles in optimizing metabolic pathways, balancing metabolic fluxes, and ultimately accelerating the development of efficient microbial cell factories for sustainable bioproduction [67]. The ongoing integration of automated biofoundries with advanced ML algorithms promises to further enhance DBTL cycle efficiency, potentially enabling fully autonomous strain optimization in the near future [71].
In metabolic engineering, the Design-Build-Test-Learn (DBTL) cycle is a foundational framework for developing robust microbial cell factories. While often perceived as an iterative process of small, incremental steps, a compelling strategy involves initiating development with a large, comprehensive cycle. This in-depth technical guide explores the rationale and methodology behind this approach, framing it within the broader thesis of the DBTL cycle's role in metabolic engineering research. We detail how a substantial initial investment in the "Design" and "Build" phases, encompassing extensive literature mining and high-throughput construction of variant libraries, can generate a rich, foundational dataset. This dataset dramatically accelerates the "Learn" phase, enabling the training of more predictive models and ultimately leading to a more efficient and successful strain optimization trajectory. The principles are illustrated with a contemporary case study on the optimized production of dopamine in Escherichia coli [3].
Systems metabolic engineering integrates tools from synthetic biology, enzyme engineering, and omics technologies to optimize microbial hosts for the sustainable production of valuable compounds [5]. The DBTL cycle provides a structured, iterative framework for this optimization [3] [72].
- Design: In this initial phase, engineering targets are selected using computational models, prior knowledge, or hypothesis-driven approaches. This involves designing genetic constructs, such as promoters, ribosome binding sites (RBS), and gene pathways, to modulate enzyme expression levels and channel metabolic flux toward the desired product [3].
- Build: The designed genetic constructs are assembled into the host organism's genome or plasmids using advanced, often automated, molecular biology and genetic engineering tools [3] [72].
- Test: The constructed microbial strains are cultivated and rigorously analyzed to measure performance metrics such as product titer, yield, and productivity. This phase relies on analytical chemistry and high-throughput screening methods [3].
- Learn: Data from the test phase is analyzed using statistical methods or machine learning. The insights gained inform the hypotheses and designs for the next DBTL cycle, creating a continuous feedback loop for strain improvement [5] [3].
A significant challenge in the DBTL cycle is the initial "knowledge gap" of the first cycle, which traditionally starts with limited prior information, potentially leading to several time- and resource-intensive iterations [3].
The Rationale for a Large Initial Cycle
Adopting a strategy that employs a large and comprehensive initial DBTL cycle can mitigate the initial knowledge gap and compress the overall development timeline. This approach is characterized by a substantial investment in the "Design" and "Build" phases to create a vast and diverse library of genetic variants for the first "Test" and "Learn" phases.
Overcoming the Initial Knowledge Barrier
Traditional DBTL cycles may select engineering targets via design of experiment or randomized selection, which can lead to numerous iterations [3]. A large initial cycle, in contrast, embraces a "knowledge-driven" approach from the outset. By generating a massive dataset in the first round, researchers can move from a state of low information to a state of high understanding much more rapidly. This foundational knowledge provides mechanistic insights that guide all subsequent, more targeted, cycles [3].
Accelerating the Learning Trajectory
The core benefit of this strategy lies in the quality of the learning phase. A larger and more diverse initial dataset allows for the application of sophisticated machine learning models to identify non-obvious correlations and design rules. For instance, testing a wide range of RBS sequences with varying Shine-Dalgarno sequences and GC content can reveal precise sequence-function relationships that would be impossible to deduce from a handful of variants [3]. This leads to more predictive models and more intelligent designs in the next cycle.
Economic and Temporal Efficiency
While a large initial cycle requires greater upfront investment in resources and automation, it can be more cost-effective overall. The alternative—multiple, sequential, small-scale DBTL cycles—incurs repeated costs associated with DNA synthesis, cloning, and personnel time. Streamlining the discovery process into fewer, more decisive cycles, as demonstrated by automated biofoundries, reduces long-term development time and costs [3] [72].
Case Study: Knowledge-Driven DBTL for Dopamine Production inE. coli
A recent study exemplifies the successful implementation of a knowledge-driven DBTL cycle for optimizing dopamine production, resulting in a 2.6 to 6.6-fold improvement over the state-of-the-art [3].
Experimental Workflow and Design
The research aimed to develop a highly efficient dopamine production strain in E. coli FUS4.T2, a host engineered for high L-tyrosine precursor supply. The synthetic pathway comprised two key enzymes: 4-hydroxyphenylacetate 3-monooxygenase (HpaBC) for converting L-tyrosine to L-DOPA, and L-DOPA decarboxylase (Ddc) from Pseudomonas putida for converting L-DOPA to dopamine [3].
The strategy involved a crucial upstream, in vitro investigation before the first in vivo DBTL cycle. This "knowledge-driven" step used a crude cell lysate system to test different relative expression levels of HpaBC and Ddc, bypassing whole-cell constraints to rapidly identify optimal enzyme ratios [3].
Build and Test Methodologies
- Library Construction (Build): The insights from the in vitro studies were translated into an in vivo environment through high-throughput RBS engineering. A library of RBS sequences was constructed, focusing on modulating the Shine-Dalgarno sequence to fine-tune the translation initiation rates of HpaBC and Ddc without altering secondary structures [3].
- Strain Cultivation and Analysis (Test): The strain library was cultivated in a defined minimal medium. Key cultivation conditions are summarized in Table 1. Dopamine production was quantified using high-performance liquid chromatography (HPLC) to identify high-performing strains [3].
Table 1: Cultivation Conditions for Dopamine Production Strains [3]
| Parameter | Specification |
|---|---|
| Host Strain | E. coli FUS4.T2 |
| Medium | Minimal medium with 20 g/L glucose, 10% 2xTY, MOPS buffer |
| Inducer | Isopropyl β-d-1-thiogalactopyranoside (IPTG), 1 mM |
| Antibiotics | Ampicillin (100 µg/mL), Kanamycin (50 µg/mL) |
| Key Supplements | 50 µM Vitamin B6, 0.2 mM FeCl₂, Trace elements |
Key Findings and Learning Outcomes
The initial large-scale DBTL cycle yielded two critical outcomes:
- High-Producing Strain: The development of a dopamine production strain achieving 69.03 ± 1.2 mg/L, equivalent to 34.34 ± 0.59 mg/gbiomass [3].
- Mechanistic Insight: The study demonstrated that the GC content within the Shine-Dalgarno sequence is a key determinant of RBS strength and, consequently, pathway performance [3].
Table 2: Performance Comparison of Dopamine Production in E. coli [3]
| Production Strain / Strategy | Dopamine Titer (mg/L) | Dopamine Yield (mg/gbiomass) |
|---|---|---|
| State-of-the-art (prior to study) | 27 | 5.17 |
| Knowledge-driven DBTL cycle | 69.03 ± 1.2 | 34.34 ± 0.59 |
| Fold-Improvement | ~2.6x | ~6.6x |
Essential Research Reagent Solutions
The following table details key materials and reagents used in the featured case study and broader metabolic engineering DBTL workflows [3].
Table 3: Research Reagent Solutions for DBTL Cycles in Metabolic Engineering
| Reagent / Material | Function in the Workflow |
|---|---|
| pET / pJNTN Plasmid Systems | Storage vectors and backbones for heterologous gene expression and library construction. |
| Ribosome Binding Site (RBS) Libraries | High-throughput fine-tuning of gene expression levels in a polycistronic pathway. |
| E. coli FUS4.T2 Production Host | An L-tyrosine overproduction chassis strain, engineered to provide high precursor flux. |
| HpaBC (4-hydroxyphenylacetate 3-monooxygenase) | A native E. coli enzyme that catalyzes the conversion of L-tyrosine to L-DOPA. |
| Ddc (L-DOPA decarboxylase) from P. putida | A heterologous enzyme that catalyzes the decarboxylation of L-DOPA to dopamine. |
| Crude Cell Lysate System | An in vitro platform for rapid prototyping of pathways and enzyme ratios without cellular regulation. |
| Automated DNA Synthesis Platform (e.g., BioXp) | Enables hands-free, rapid synthesis of DNA constructs, drastically shortening the "Build" phase [72]. |
Visualizing the Dopamine Biosynthetic Pathway
The two-step heterologous pathway engineered into E. coli for dopamine production is illustrated below.
The strategy of deploying a large initial DBTL cycle, supported by upstream knowledge gathering and high-throughput automation, represents a paradigm shift in metabolic engineering. It moves the field away from slow, iterative guessing and towards rapid, mechanistic-driven strain optimization. As demonstrated by the successful development of a high-yielding dopamine strain, this approach can significantly accelerate the design of microbial cell factories for a wide range of valuable biochemicals, aligning with the growing demands of sustainable biomanufacturing.
Integrating Mechanistic and Data-Driven Models for Enhanced Predictive Power
The Design-Build-Test-Learn (DBTL) cycle is a systematic, iterative framework central to modern metabolic engineering and synthetic biology, enabling the rational development and optimization of microbial cell factories [46] [71]. In this framework, "Design" involves planning genetic modifications; "Build" is the implementation of these designs in a host organism; "Test" characterizes the performance of the engineered strain; and "Learn" analyzes the collected data to inform the next design iteration [9]. The integration of mechanistic models and data-driven machine learning (ML) represents a powerful evolution of this cycle. Mechanistic models, grounded in biochemical principles, provide a interpretable representation of cellular metabolism. In contrast, ML models can uncover complex, non-intuitive patterns from high-dimensional data. Their combined use creates a synergistic loop where mechanistic insights constrain and inform ML models, which in turn can refine and validate mechanistic hypotheses, leading to significantly enhanced predictive power for optimizing bioproduction processes [1] [73].
The DBTL Cycle: A Detailed Framework
Core Phases of the DBTL Cycle
The DBTL cycle's power lies in its structured, iterative approach to strain engineering. The table below details the objectives and key activities for each phase.
Table 1: Core Phases of the Design-Build-Test-Learn Cycle
| Phase | Primary Objective | Key Activities & Methodologies |
|---|---|---|
| Design | To plan genetic interventions for optimizing metabolic pathways. | In silico pathway design using tools like RetroPath [9]; Combinatorial library design using promoter/RBS engineering [1] [3]; Design of Experiments (DoE) for library reduction [9]. |
| Build | To physically construct the designed genetic variants in a microbial host. | Automated DNA assembly (e.g., Ligase Cycling Reaction) [9]; High-throughput cloning; Genome editing tools (e.g., MAGE) [71]. |
| Test | To characterize the performance of engineered strains (titer, yield, rate). | Cultivation in microplates or bioreactors [9]; Analytics (e.g., LC-MS/MS) for metabolites [9]; Omics data acquisition (transcriptomics, proteomics) [71]. |
| Learn | To extract insights from experimental data to guide the next design. | Statistical analysis to identify key performance factors [9]; Machine learning model training on experimental data [1] [73]; Mechanistic model simulation and refinement [1]. |
Visualizing the Workflow and Its Evolution
The following diagram illustrates the standard DBTL cycle and the integrated role of mechanistic and data-driven models.
A paradigm shift termed "LDBT" (Learn-Design-Build-Test) has been proposed, where machine learning, powered by large pre-existing datasets, precedes the design phase [74]. This approach leverages zero-shot predictions from protein language models and other AI tools to generate initial designs, potentially reducing the number of iterative cycles required.
Mechanistic Models in the DBTL Cycle
Fundamentals and Implementation
Mechanistic models in metabolic engineering are typically based on kinetic modeling, where changes in intracellular metabolite concentrations are described by ordinary differential equations (ODEs) derived from biochemical reaction mechanisms and mass action kinetics [1]. These models explicitly represent enzyme concentrations, catalytic rates, and regulatory interactions, allowing for in silico perturbation of pathway elements, such as changing enzyme expression levels, to predict their effect on metabolic flux and product formation [1]. A key application is creating a mechanistic framework for benchmarking ML methods. By simulating a metabolic pathway embedded in a physiologically relevant cell model (e.g., an E. coli core kinetic model), researchers can generate in-silico "data" for multiple DBTL cycles, enabling systematic comparison of different ML algorithms without the cost and time of real-world experiments [1].
A Worked Example: Simulated Pathway Optimization
A demonstrated workflow involves integrating a synthetic pathway into a core kinetic model of E. coli [1]. The pathway, designed to maximize the production of a target compound, is subjected to combinatorial perturbations of enzyme levels (simulating promoter/RBS libraries). The kinetic model simulates the outcome (e.g., product flux) for each variant. This simulated DBTL cycle allows for the testing of ML models in a controlled environment, revealing, for instance, that gradient boosting and random forest models outperform other methods in low-data regimes and are robust to experimental noise [1].
Data-Driven Machine Learning Models in the DBTL Cycle
Machine Learning Approaches and Applications
Machine learning brings the ability to learn complex, non-linear relationships from multi-omics data and high-throughput screening results, which is often intractable for purely mechanistic models.
Table 2: Machine Learning Models for Metabolic Engineering
| ML Category | Example Models | Key Applications in DBTL | References |
|---|---|---|---|
| Supervised Learning | Gradient Boosting, Random Forest, Support Vector Machines (SVMs) | Predicting strain performance from genetic design; Recommending new strain designs for the next DBTL cycle. | [1] [73] |
| Protein Language Models | ESM, ProGen, ProteinMPNN, MutCompute | Zero-shot design of enzyme variants with improved stability or activity; Predicting functional mutations. | [74] |
| Specialized Predictors | Prethermut, Stability Oracle, DeepSol | Predicting protein thermostability (ΔΔG) and solubility from sequence or structure. | [74] |
| Neural Networks | Graph Neural Networks (GNNs), Physics-Informed Neural Networks (PINNs) | Learning from complex biological networks; Incorporating physical constraints into data-driven models. | [71] |
Recommendation Algorithms for DBTL Cycling
A critical application of ML is the development of automated recommendation tools. These tools use an ensemble of ML models to create a predictive distribution of strain performance across the unexplored design space. Based on this distribution and a user-defined exploration/exploitation parameter, the algorithm samples and recommends a new set of strain designs to build and test in the subsequent DBTL cycle [1]. This facilitates (semi)-automated iterative metabolic engineering.
Integrated Methodologies and Experimental Protocols
Protocol: Kinetic Model-Guided DBTL Benchmarking
This protocol outlines the steps for using a mechanistic kinetic model to simulate DBTL cycles and benchmark machine learning algorithms [1].
- Model Construction: Implement a kinetic model of a host organism (e.g., the E. coli core metabolism) and integrate the target synthetic pathway. The model should include reactions for biomass formation and product synthesis.
- Parameterization: Use computational sampling techniques (e.g., ORACLE) to generate thermodynamically feasible kinetic parameter sets that reflect physiological states [1].
- Define Design Space: Specify a combinatorial library of genetic perturbations (e.g., 5 discrete enzyme expression levels for each of 5 pathway enzymes, creating 3125 possible designs).
- In-silico "Build & Test": Simulate the model for each design variant to generate a comprehensive dataset of enzyme expression levels (input) and product flux/titer (output).
- ML Training & Benchmarking:
- Sample a subset of the full dataset to represent an initial experimental DBTL cycle.
- Train multiple ML models (e.g., Random Forest, Gradient Boosting) on this subset.
- Use a recommendation algorithm to select the next set of strains to "build."
- Iterate the cycle and compare the performance of different ML models in efficiently navigating the design space towards the global optimum.
Protocol: Automated DBTL for Flavonoid Production
This protocol summarizes an automated DBTL pipeline applied to optimize (2S)-pinocembrin production in E. coli [9].
- Design:
- Enzyme Selection: Use in silico tools (RetroPath, Selenzyme) to select enzymes for the pathway.
- Combinatorial Library Design: Design a library varying parameters like vector copy number, promoter strength, and gene order. For a 4-gene pathway, this can generate thousands of combinations.
- Library Reduction: Apply Design of Experiments (DoE), such as orthogonal arrays, to reduce the library to a tractable, representative subset (e.g., from 2592 to 16 constructs).
- Build:
- Automated DNA Assembly: Use robotic platforms and standardized assembly methods (e.g., Ligase Cycling Reaction) to construct the pathway plasmids.
- Quality Control: Perform high-throughput plasmid purification, restriction digest, and sequencing to verify constructs.
- Test:
- Cultivation: Grow engineered strains in 96-deepwell plates under controlled conditions.
- Analytics: Use automated extraction and quantitative UPLC-MS/MS to measure titers of the target product and key intermediates.
- Learn:
- Statistical Analysis: Identify the main factors (e.g., plasmid copy number, promoter strength for specific genes) significantly influencing product titer using statistical tests (e.g., ANOVA).
- Redesign: Use these findings to constrain the design space for the next DBTL cycle, focusing on the most impactful factors.
The Scientist's Toolkit: Essential Research Reagents and Solutions
Table 3: Key Research Reagents and Materials for DBTL Experiments
| Item | Function / Application | Example Use Case |
|---|---|---|
| Ribosome Binding Site (RBS) Libraries | Fine-tuning the translation initiation rate and relative expression levels of pathway enzymes. | Optimizing the flux balance in a dopamine or pinocembrin biosynthetic pathway [3] [9]. |
| Promoter Libraries | Transcriptional-level control of gene expression (e.g., constitutive, inducible). | Varying enzyme concentrations to identify and overcome rate-limiting steps [1] [9]. |
| Cell-Free Protein Synthesis (CFPS) Systems | Rapid in vitro prototyping of pathway enzymes and pathway combinations without the constraints of a living cell. | Accelerating the Build-Test phases for initial pathway validation and generating large training datasets for ML [74]. |
| Ligase Cycling Reaction (LCR) Reagents | An automated, robust method for the assembly of multiple DNA parts into a single plasmid. | High-throughput construction of genetic variant libraries in the Build phase [9]. |
| UPLC-MS/MS Systems | High-resolution, sensitive quantification of metabolites, products, and pathway intermediates from culture broth. | Providing high-quality, quantitative data for the Test phase and for training ML models [9]. |
The integration of mechanistic and data-driven models within the DBTL cycle marks a significant leap forward for metabolic engineering. Mechanistic models provide a foundational understanding and a sandbox for in silico testing, while machine learning excels at extracting actionable insights from complex, high-dimensional data. Their synergy creates a powerful, iterative feedback loop that enhances predictive power, guides exploration, and accelerates the rational design of high-performing microbial cell factories. Emerging trends like the LDBT paradigm and the use of cell-free systems for ultra-high-throughput data generation are poised to further reduce development timelines, pushing the field closer to a fully predictive and automated engineering discipline.
The Design-Build-Test-Learn (DBTL) cycle serves as the fundamental engineering framework in synthetic biology and metabolic engineering for developing biological systems with enhanced functions [12]. This iterative process begins with Design, where researchers define objectives and design biological parts using computational tools and domain knowledge. The Build phase involves the physical construction of these designs, typically through DNA synthesis and assembly into host organisms. The Test phase characterizes the performance of the built constructs, and the Learn phase analyzes the resulting data to inform the next design iteration [74]. As metabolic engineering ambitions grow more complex—targeting the production of advanced biofuels, therapeutics, and sustainable chemicals—the limitations of current DNA synthesis capabilities have created a critical bottleneck in the Build phase that impacts the entire DBTL cycle efficiency [75] [76].
The Build-Phase Bottleneck: Limitations of Current DNA Synthesis Technologies
The DNA Writing Gap
While DNA sequencing (reading) technologies have advanced rapidly, DNA synthesis (writing) capabilities have lagged significantly, creating what is known as the "DNA writing gap" [75]. Traditional phosphoramidite chemistry, the dominant synthesis method for decades, faces fundamental limitations that restrict its ability to produce the long, complex DNA sequences required for modern metabolic engineering projects. This chemical synthesis approach suffers from sub-99.5% per-step coupling efficiencies, causing an exponential drop in yield with increasing sequence length [76]. Sequences beyond approximately 200 bases typically yield low amounts of correct product dominated by deletion errors and truncations [76].
Table 1: Quantitative Comparison of DNA Synthesis Technologies
| Synthesis Method | Maximum Length (bases) | Coupling Efficiency | Key Limitations | Error Rate |
|---|---|---|---|---|
| Traditional Chemical (Phosphoramidite) | ~200 | <99.5% | Sequence complexity sensitivity, hazardous waste | G-to-A: 0.01-0.1% [77] |
| Enzymatic DNA Synthesis (EDS) | 500+ (services), 120+ (benchtop) | >99.5% | Emerging technology, cost | Significantly reduced for complex sequences [76] |
Sequence Complexity Challenges
Metabolic engineering projects frequently require DNA sequences with complex structural elements that are particularly challenging for conventional synthesis methods. Key problematic sequences include:
- High GC content (>65%) and stable secondary structures [76]
- Long repetitive sequences such as CRISPR arrays [78]
- Inverted terminal repeats (ITRs) critical for AAV vector transcription [76]
- Structured untranslated regions (UTRs) and fixed-length poly A tails in mRNA constructs [78] [76]
These challenging sequences often cause synthetic failures or require extensive troubleshooting, significantly delaying DBTL cycling times [76]. For instance, the palindromic nature of ITRs makes them notoriously difficult to synthesize chemically with the fidelity required for safe and effective gene delivery vectors [76].
Technological Innovations: Enzymatic DNA Synthesis and Error Reduction
Enzymatic DNA Synthesis (EDS)
Enzymatic DNA synthesis (EDS) represents a paradigm shift from traditional chemical methods by using biological catalysts instead of harsh chemicals [76]. This approach employs engineered versions of terminal deoxynucleotidyl transferase (TdT) in a template-independent manner to add nucleotides sequentially to a growing DNA chain [75] [76]. Key advantages include:
- Mild aqueous conditions near physiological pH and temperature that reduce DNA damage [76]
- Reduced sensitivity to sequence complexity due to hybridization-independent mechanisms [76]
- Drastically reduced generation of hazardous waste compared to traditional methods [76]
- Superior capability for synthesizing complex sequences including those with high GC content and secondary structures [76]
Internal benchmarking at DNA Script has demonstrated that sequences often considered 'unmanufacturable'—including fragments from 1.5 kb to 7 kb with challenging structural features—can be successfully synthesized and assembled using EDS oligonucleotides [76].
Error Suppression Methodologies
Recent research has quantified synthetic errors and developed effective suppression strategies. Comprehensive error analysis using next-generation sequencing has identified that G-to-A substitutions are the most prominent errors in chemical synthesis, influenced significantly by capping conditions during synthesis [77]. Innovative approaches using non-canonical nucleosides such as 7-deaza-2´-deoxyguanosine and 8-aza-7-deaza-2´-deoxyguanosine as error-proof alternatives have demonstrated a 50-fold decrease in G-to-A substitution error rates when phenoxyacetic anhydride was used as capping reagents [77].
Diagram 1: DBTL cycle with build limitations
Impact on Metabolic Engineering Applications
Biofuel Production
Advanced biofuel production exemplifies how DNA synthesis limitations impact metabolic engineering outcomes. Fourth-generation biofuels utilize genetically modified (GM) algae and photobiological solar fuels with engineered metabolic pathways for improved photosynthetic efficiency and enhanced lipid accumulation [79]. These systems require precisely synthesized pathways for producing hydrocarbons, isoprenoids, and jet fuel analogs that are fully compatible with existing infrastructure [79]. The complexity of these multi-enzyme pathways demands high-fidelity long DNA synthesis that often exceeds conventional capabilities.
Therapeutic Development
The therapeutic sector faces similar challenges, with mRNA vaccines, cell and gene therapies, and genetic medicines requiring increasingly complex DNA templates [78] [76]. For example, optimal mRNA vaccine design necessitates long DNA templates (many kilobases) incorporating intricate untranslated regions (UTRs) with GC-rich motifs and complex secondary structures crucial for mRNA stability and translational efficiency [76]. The inability to reliably access these complex sequences hampers innovation across critical therapeutic areas [76].
Table 2: DNA-Dependent Applications in Metabolic Engineering and Therapeutics
| Application Area | DNA Requirements | Synthesis Challenges | Impact of Improved Synthesis |
|---|---|---|---|
| Advanced Biofuels [79] | Multi-gene pathways for hydrocarbon production | Long constructs with complex regulatory elements | Higher yield drop-in fuels |
| mRNA Therapeutics [76] | DNA templates with optimized UTRs | GC-rich regions, secondary structures | Improved vaccine efficacy and stability |
| AAV Gene Therapies [76] | Inverted terminal repeats (ITRs) | Palindromic sequences, secondary structures | Accelerated vector development |
| Antibody Engineering [76] | Large variant libraries, bispecific formats | Repetitive sequences, long fragments | Faster discovery pipelines |
Experimental Protocols for DNA Synthesis Quality Control
Error Quantification Using Next-Generation Sequencing
Comprehensive quality assessment of synthetic DNA requires precise error quantification protocols:
Library Preparation Method [77]:
- Design: Create reference sequences avoiding single nucleotide repeats but including all 12 other dimer combinations
- Assembly: Use polymerase-based assembling reaction rather than ligation to prepare NGS libraries
- Sequencing: Perform paired-end sequencing on next-generation platforms
- Data Processing:
- Merge paired-end reads
- Omit sequences containing N-base calls or base calls with Q score <40
- Perform alignment to reference sequence using Needleman-Wunsch aligner
- Calculate error rates for substitution, insertion, and deletion at each sequence position
Polymerase Selection Considerations [77]:
- High-fidelity polymerases (Q5, Phusion) recommended for accurate error detection
- Standard polymerases (Ex Taq) may show different error profiles due to differential recognition of unnatural nucleobases
Cell-Free Prototyping for DBTL Acceleration
Integrating cell-free systems with DNA synthesis creates powerful workflows for rapid DBTL cycling:
iPROBE (in vitro Prototyping and Rapid Optimization of Biosynthetic Enzymes) Methodology [74]:
- Cell-free transcription-translation: Use crude cell lysates or purified components for protein expression
- Pathway prototyping: Test pathway combinations and enzyme expression levels without cloning
- Machine learning integration: Train neural networks on pathway performance data to predict optimal configurations
- Validation: Implement top predictions in vivo, achieving >20-fold improvements in target compounds [74]
Diagram 2: DNA synthesis methods comparison
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Reagents for DNA Synthesis and Quality Control
| Reagent/Technology | Function | Application Context |
|---|---|---|
| Terminal Deoxynucleotidyl Transferase (TdT) [75] [76] | Template-independent enzymatic DNA synthesis | EDS platforms for complex sequence synthesis |
| Error-Proof Nucleosides (7-deaza-2´-deoxyguanosine) [77] | Reduce G-to-A substitution errors | High-fidelity oligonucleotide synthesis |
| Phenoxyacetic Anhydride [77] | Capping reagent for error suppression | Chemical synthesis with reduced error rates |
| Q5 High-Fidelity DNA Polymerase [77] | Error quantification in synthetic oligonucleotides | NGS library preparation for quality control |
| Cell-Free Transcription-Translation Systems [74] | Rapid pathway prototyping without cloning | DBTL acceleration before in vivo implementation |
| Non-canonical Nucleosides [77] | Resistance to synthesis side reactions | Improved sequence quality in genome synthesis |
The paradigm of DBTL cycles in metabolic engineering is evolving toward more integrated approaches. Emerging frameworks propose LDBT (Learn-Design-Build-Test) cycles where machine learning precedes design, leveraging large biological datasets to make zero-shot predictions that potentially eliminate multiple DBTL iterations [74]. The success of such approaches depends fundamentally on the ability to rapidly and reliably build predicted sequences, highlighting the continued critical importance of advancing DNA synthesis technologies [74].
Enzymatic DNA synthesis continues to evolve with improvements in synthesis speed, achievable length, sequence fidelity, and cost-effectiveness [76]. These advancements position EDS as a crucial enabling technology for overcoming synthesis bottlenecks that currently impede discovery and development across metabolic engineering applications [76]. Additionally, fully enzymatic synthesis methods contribute to greener biotechnology by reducing dependence on chemical reagents and organic solvents with adverse environmental impacts [75].
As metabolic engineering tackles increasingly ambitious projects—from sustainable chemical production to advanced therapeutics—addressing the build-phase limitations through high-quality, long DNA synthesis will remain a critical frontier. The integration of enzymatic synthesis technologies with machine learning-guided design and rapid cell-free testing creates a powerful foundation for the next generation of DBTL cycles, potentially transforming synthetic biology from an iterative engineering discipline to a more predictive science capable of addressing pressing global challenges.
DBTL Cycle Validation: Case Studies, Comparative Performance, and Future Directions
The Design-Build-Test-Learn (DBTL) cycle represents a systematic, iterative framework for engineering biological systems, particularly in optimizing microbial cell factories for biochemical production [5] [71]. In metabolic engineering, this approach enables the progressive development of strains with enhanced product titers, yields, and productivity by repeatedly designing genetic modifications, building strains, testing their performance, and learning from the results to inform the next cycle [9]. The traditional DBTL process, however, faces significant challenges in terms of time, cost, and experimental effort, especially when tackling combinatorial pathway optimization where testing all possible genetic combinations becomes infeasible [1].
Recent advances have introduced computational frameworks to enhance the efficiency of DBTL cycling, with kinetic model-based approaches emerging as particularly powerful validation tools [1] [80]. These simulated DBTL cycles create a mechanistic representation of metabolic pathways embedded in physiologically relevant cell models, allowing researchers to test and optimize machine learning methods and experimental strategies before committing to costly wet-lab experiments [1]. This guide explores the implementation, validation, and application of kinetic model-based frameworks for simulating DBTL cycles in metabolic engineering research.
The Kinetic Modeling Framework for DBTL Simulations
Core Components and Structure
The kinetic model-based framework for simulating DBTL cycles employs mechanistic kinetic models to represent metabolic pathways and their interactions with host cell physiology [1]. This approach uses ordinary differential equations (ODEs) to describe changes in intracellular metabolite concentrations over time, with each reaction flux governed by kinetic mechanisms derived from mass action principles [1]. This biological relevance enables in silico manipulation of pathway elements, such as modifying enzyme concentrations or catalytic properties, to simulate genetic engineering interventions.
The framework integrates several key components:
- Pathway Representation: A synthetic pathway is embedded within an established core kinetic model of Escherichia coli metabolism, implemented using the Symbolic Kinetic Models in Python (SKiMpy) package [1]
- Combinatorial Design Space: The framework simulates libraries of genetic components (promoters, RBS sequences, coding sequences) that affect enzyme expression levels and activity [1]
- Bioprocess Integration: The cellular model is embedded within a bioreactor system simulating batch fermentation processes with biomass growth, substrate consumption, and product formation [1]
Simulating Metabolic Pathway Behavior
The kinetic model captures non-intuitive pathway behaviors that complicate traditional sequential optimization approaches [1]. For example, perturbations to individual enzyme concentrations may have counterintuitive effects on metabolic flux due to complex pathway interactions and substrate depletion effects [1]. The table below illustrates how simulated enzyme perturbations affect reaction fluxes and product formation:
Table 1: Effects of Simulated Enzyme Perturbations on Metabolic Flux
| Enzyme Perturbed | Effect on Respective Reaction Flux | Effect on Product Flux | Interpretation |
|---|---|---|---|
| Enzyme A | No significant change | 1.5-fold increase | Non-intuitive coupling effects |
| Enzyme B | Decreased flux (substrate depletion) | No significant change | Metabolic bottleneck |
| Enzyme G (final step) | Decreased flux | Increased net production | Reduced downstream drain |
These simulated behaviors demonstrate why combinatorial optimization is essential for pathway engineering, as sequential optimization strategies often miss global optimum configurations of pathway elements [1]. The kinetic model effectively captures the emergent properties that result from multiple simultaneous perturbations, providing a realistic testbed for DBTL cycle optimization.
Implementing Simulated DBTL Cycles
The Simulation Workflow
The simulated DBTL cycle follows a structured workflow that mirrors experimental strain engineering while operating entirely in silico. This process enables researchers to systematically evaluate different machine learning approaches and experimental strategies for combinatorial pathway optimization.
Machine Learning Integration and Performance
The Learn phase of simulated DBTL cycles employs machine learning (ML) algorithms to predict strain performance from previous cycles and recommend designs for subsequent iterations [1]. The framework enables systematic comparison of different ML methods across multiple simulated cycles, addressing a significant challenge in experimental metabolic engineering where such comparisons are rarely feasible due to resource constraints [1].
Table 2: Machine Learning Method Performance in Simulated DBTL Cycles
| ML Method | Performance in Low-Data Regime | Robustness to Training Bias | Robustness to Experimental Noise | Key Applications |
|---|---|---|---|---|
| Gradient Boosting | Top performer | High | High | Genotype-phenotype predictions, design recommendation |
| Random Forest | Top performer | High | High | Feature importance analysis, phenotype prediction |
| SGD Regressor | Moderate | Moderate | Moderate | Large-scale datasets, linear relationships |
| MLP Regressor | Lower | Variable | Variable | Complex nonlinear relationships |
| Automated Recommendation Tool | Variable | Dependent on base models | Dependent on base models | Balancing exploration/exploitation in design selection |
The simulated framework demonstrates that gradient boosting and random forest models consistently outperform other methods in the low-data regime typical of early DBTL cycles, while maintaining robustness to training set biases and experimental noise [1]. These algorithms effectively learn complex relationships between genetic modifications and metabolic flux, enabling increasingly informed design selections with each cycle.
Experimental Protocols and Methodologies
Kinetic Model Development Protocol
Developing a kinetic model for DBTL simulation requires careful construction and parameterization to ensure biological relevance:
- Pathway Definition: Identify target metabolic pathway and integrate into host core metabolic model
- Kinetic Parameterization: Sample kinetic parameters using ORACLE sampling to ensure thermodynamic feasibility [1]
- Enzyme Modulation: Implement enzyme expression changes by modifying Vmax parameters proportional to promoter strength or RBS variations [1]
- Bioprocess Modeling: Embed cellular model into bioreactor system simulating batch fermentation conditions [1]
- Validation: Verify model produces physiologically realistic behavior including biomass growth, substrate consumption, and product formation
Simulated DBTL Cycle Execution
Executing simulated DBTL cycles follows a structured protocol:
- Combinatorial Space Generation: Enumerate all possible genetic designs from available parts library [81]
- Initial Design Selection: Choose initial training set using specified sampling strategy (random, biased, or DoE-based) [1]
- Phenotype Simulation: Calculate product titers for each design using kinetic model [81]
- ML Model Training: Train machine learning models on simulated design-performance data [1]
- Design Recommendation: Apply recommendation algorithm to select designs for next cycle based on exploration-exploitation balance [1]
- Cycle Iteration: Repeat steps 3-5 for multiple DBTL cycles, tracking performance metrics [81]
Performance Evaluation Metrics
The framework employs multiple metrics to evaluate DBTL cycle performance:
- Prediction Accuracy: R² values between predicted and actual product titers [81]
- Top Producer Identification: Intersection score measuring recovery of top-performing strains [81]
- Convergence Rate: Number of cycles required to reach performance targets [1]
- Efficiency: Total number of strains simulated to achieve optimization goal [1]
Key Research Reagents and Computational Tools
Implementing simulated DBTL cycles requires specific computational tools and frameworks that form the essential "research reagents" for in silico metabolic engineering.
Table 3: Essential Research Reagents and Computational Tools
| Tool/Platform | Type | Function in DBTL Framework | Application Example |
|---|---|---|---|
| SKiMpy | Software package | Kinetic modeling and simulation | Building mechanistic models of metabolic pathways [1] |
| JAXKineticModel | Computational library | Kinetic model implementation | Custom pathway integration and simulation [81] |
| scikit-learn | ML library | Machine learning algorithms | Gradient boosting, random forest implementation [1] |
| TeselaGen | Platform | DBTL cycle management | End-to-end workflow support with AI integration [26] |
| PySBOL | Standardized API | Workflow data management | Tracking Designs, Builds, Tests, and Analyses [82] |
| AbeelLab GitHub Repository | Code repository | Framework implementation | Reproducing simulated DBTL experiments [81] |
Application Case Studies and Validation
DBTL Cycle Strategy Optimization
The kinetic model framework enables systematic comparison of different DBTL cycle strategies that would be impractical to test experimentally. Research demonstrates that when the total number of strains is limited, starting with a larger initial DBTL cycle produces better outcomes than distributing the same number of strains evenly across cycles [1]. This strategy provides more comprehensive initial data for machine learning models, enhancing their predictive accuracy in subsequent cycles.
The framework also evaluates different sampling approaches for initial design selection:
- Equal Sampling: Uniform sampling across all enzyme expression levels
- Radical Sampling: Biased toward extreme expression levels (very high or very low)
- Non-radical Sampling: Biased toward moderate expression levels near wild-type
Results indicate that ML methods maintain robust performance across these sampling biases, though equal sampling generally provides the most comprehensive exploration of the design space [1].
Pathway Optimization for Biochemical Production
The simulated DBTL framework has been applied to optimize pathways for various biochemicals, including C5 platform chemicals derived from L-lysine in Corynebacterium glutamicum [5]. In these applications, the kinetic model captures complex interactions within the metabolic network, enabling identification of optimal enzyme expression ratios that maximize flux toward target compounds while minimizing metabolic burden [5].
Another application demonstrates optimization of dopamine production in E. coli, where a knowledge-driven DBTL cycle combined upstream in vitro investigation with high-throughput RBS engineering to achieve a 2.6 to 6.6-fold improvement over state-of-the-art production [3]. This approach provided mechanistic insights into how GC content in the Shine-Dalgarno sequence influences translation initiation rates and pathway efficiency.
Future Directions and Implementation Considerations
Framework Extensions and Enhancements
Future developments in kinetic model-based DBTL simulation include:
- Multi-scale Modeling: Integrating kinetic models with regulatory networks and host physiology
- Automated Experimental Design: Using digital twins to guide biofoundry operations [71]
- Transfer Learning: Applying knowledge from simulated to experimental DBTL cycles
- Hybrid Modeling: Combining mechanistic models with machine learning surrogates
Practical Implementation Guidelines
For research teams implementing simulated DBTL frameworks:
- Start with Well-Characterized Pathways: Begin validation with pathways having established kinetic parameters
- Calibrate with Experimental Data: Where possible, use limited experimental data to validate model predictions
- Iterate Model Complexity: Begin with simplified representations, increasing complexity as needed
- Validate with Multiple Metrics: Assess framework performance using both prediction accuracy and optimization efficiency
The kinetic model-based approach for simulating DBTL cycles represents a powerful methodology for accelerating metabolic engineering efforts, reducing experimental costs, and providing insights into optimal strain design strategies. By creating a digital twin of the metabolic optimization process, researchers can explore design spaces more comprehensively and develop more effective ML-guided engineering strategies before committing to laboratory experiments.
The Design-Build-Test-Learn (DBTL) cycle is a systematic framework central to modern metabolic engineering and synthetic biology. It involves iteratively designing genetic modifications, building microbial strains, testing their performance, and learning from the data to inform the next design cycle [12]. This iterative process is crucial for optimizing complex biological systems, where rational design alone often fails to predict the global optimum due to non-intuitive pathway interactions and cellular regulatory mechanisms [1]. The integration of advanced tools such as automation, machine learning, and multi-omics analyses has significantly accelerated the DBTL cycle, enabling more efficient development of microbial cell factories for producing valuable chemicals [71]. This review provides a comparative analysis of strain performance achieved through DBTL-driven approaches versus state-of-the-art productions, highlighting the quantitative improvements, detailed methodologies, and essential tools that have advanced the field.
Quantitative Comparison of Production Performance
The implementation of iterative DBTL cycles has demonstrated substantial improvements in production metrics across various microbial hosts and target compounds. The table below summarizes key performance indicators from recent case studies, comparing DBTL-optimized strains with previous state-of-the-art productions.
Table 1: Performance comparison of DBTL-driven strains versus state-of-the-art productions
| Target Compound | Host Organism | State-of-the-Art Production | DBTL-Optimized Production | Fold Improvement | Key DBTL Strategy | Citation |
|---|---|---|---|---|---|---|
| Dopamine | Escherichia coli | 27 mg/L, 5.17 mg/g₍bᵢₒₘₐₛₛ₎ | 69.03 mg/L, 34.34 mg/g₍bᵢₒₘₐₛₛ₎ | 2.6-6.6 fold | Knowledge-driven DBTL with RBS engineering | [3] |
| (2S)-Pinocembrin | Escherichia coli | Not specified (baseline) | 500-fold increase, 88 mg/L | 500-fold | Automated DBTL with combinatorial library design | [9] |
| C5 Chemicals (from L-lysine) | Corynebacterium glutamicum | Varies by specific compound | Significant improvements reported | Not quantified | Systems metabolic engineering within DBTL cycle | [5] |
| Various metabolites | Corynebacterium glutamicum | Baseline from stoichiometric methods | 292% increase in precision, 106% increase in accuracy | 2.92-2.06 fold | ET-OptME framework with enzyme-thermo constraints | [83] |
Detailed Experimental Protocols in DBTL Workflows
Knowledge-Driven DBTL for Dopamine Production
A recent study demonstrated the application of a knowledge-driven DBTL cycle for optimizing dopamine production in E. coli, resulting in a 2.6 to 6.6-fold improvement over previous state-of-the-art production [3]. The methodology encompassed several key phases:
Pathway Design and In Vitro Validation: The dopamine biosynthetic pathway was constructed using the native E. coli gene encoding 4-hydroxyphenylacetate 3-monooxygenase (HpaBC) to convert L-tyrosine to L-DOPA, and heterologous L-DOPA decarboxylase (Ddc) from Pseudomonas putida to catalyze dopamine formation. Preliminary testing was conducted in a cell-free protein synthesis (CFPS) system using crude cell lysates to assess enzyme expression and functionality before moving to in vivo experiments [3].
Strain Engineering for Precursor Availability: The host strain E. coli FUS4.T2 was engineered for enhanced L-tyrosine production through deletion of the transcriptional dual regulator TyrR and mutation of the feedback inhibition in chorismate mutase/prephenate dehydrogenase (TyrA) [3].
In Vivo Fine-Tuning via RBS Engineering: A high-throughput ribosome binding site (RBS) engineering approach was implemented to optimize the relative expression levels of HpaBC and Ddc. The Shine-Dalgarno sequence was systematically modulated without interfering with secondary structures, and transformants were screened in 96-deepwell plate cultures [3].
Analytical Methods: Dopamine quantification was performed via ultra-performance liquid chromatography coupled with mass spectrometry (UPLC-MS). Biomass measurements were conducted to normalize production yields, reported as mg per gram biomass [3].
Automated DBTL for Flavonoid Production
An integrated automated DBTL pipeline was applied to optimize (2S)-pinocembrin production in E. coli, achieving a 500-fold improvement over initial designs and reaching titers of 88 mg/L [9]. The experimental workflow included:
Automated Pathway Design: Computational tools including RetroPath for pathway selection, Selenzyme for enzyme selection, and PartsGenie for DNA part design were employed. Combinatorial libraries were designed with varying parameters: four expression levels through vector backbones (varying copy number), promoter strengths (strong Ptrc or weak PlacUV5), intergenic regions with strong, weak, or no promoter, and 24 gene order permutations [9].
Library Compression and Assembly: Design of Experiments (DoE) based on orthogonal arrays combined with a Latin square for gene arrangement reduced 2592 possible combinations to 16 representative constructs. Automated ligase cycling reaction (LCR) was performed on robotics platforms for pathway assembly, followed by transformation in E. coli DH5α [9].
High-Throughput Screening: Constructs were screened in 96-deepwell plate formats with automated growth/induction protocols. Target products and intermediates were detected using fast UPLC coupled with tandem mass spectrometry with high mass resolution [9].
Statistical Analysis and Redesign: Statistical analysis of pinocembrin titers identified vector copy number as the strongest significant factor affecting production, followed by chalcone isomerase (CHI) promoter strength. This learning informed the second DBTL cycle design, which constrained the design space to specific regions showing promise [9].
ET-OptME Framework for Constraint-Based Optimization
The ET-OptME framework incorporates enzyme efficiency and thermodynamic feasibility constraints into genome-scale metabolic models, demonstrating significant improvements in prediction accuracy and precision compared to previous constraint-based methods [83]. The methodology involves:
Constraint Layering: A stepwise approach systematically incorporates enzyme abundance constraints derived from proteomics data and thermodynamic constraints based on reaction energy calculations into genome-scale metabolic models [83].
Flux Analysis Optimization: The framework utilizes advanced algorithms to mitigate thermodynamic bottlenecks and optimize enzyme usage, delivering more physiologically realistic intervention strategies compared to traditional stoichiometric methods like OptForce and FSEOF [83].
Validation Across Multiple Targets: The algorithm was quantitatively evaluated for five product targets in Corynebacterium glutamicum models, showing substantial increases in minimal precision (≥292%) and accuracy (≥106%) compared to stoichiometric methods [83].
Essential Research Reagents and Tools
The successful implementation of DBTL cycles relies on specialized research reagents and tools that enable precise genetic modifications and high-throughput screening.
Table 2: Key research reagent solutions for DBTL cycle implementation
| Reagent/Tool Category | Specific Examples | Function in DBTL Workflow | Application Example |
|---|---|---|---|
| DNA Assembly Systems | Ligase Cycling Reaction (LCR), Gibson Assembly | High-throughput pathway assembly from DNA parts | Automated construction of flavonoid pathway variants [9] |
| Vector Systems | pSEVA261, pET plasmids, pJNTN | Modular expression vectors with varying copy numbers | Medium-low copy pSEVA261 for reduced basal expression in biosensors [29] |
| Regulatory Elements | RBS libraries, Promoter variants (Ptrc, PlacUV5), Terminators | Fine-tuning gene expression levels | RBS engineering for optimizing dopamine pathway enzyme ratios [3] |
| Genome Engineering Tools | CRISPR/Cas9, MAGE, Base editors | Targeted genomic modifications | Host strain engineering for enhanced precursor availability [3] [71] |
| Analytical Instruments | UPLC-MS/MS, HRMS, Flow-injection analysis | High-throughput quantification of metabolites and products | Automated extraction and fast UPLC-MS/MS for flavonoid screening [9] |
| Bioinformatics Software | RetroPath, Selenzyme, PartsGenie, UTR Designer | In silico pathway design and part optimization | Designing combinatorial libraries for pinocembrin pathway [9] |
Workflow and Pathway Diagrams
Generic DBTL Cycle Workflow
The following diagram illustrates the iterative nature of the DBTL cycle and its key components across different applications:
Dopamine Biosynthetic Pathway
The metabolic pathway for dopamine production in engineered E. coli involves both endogenous and heterologous enzymes:
The comparative analysis of DBTL-driven strain performance versus state-of-the-art productions demonstrates the significant advantages of iterative, data-driven approaches in metabolic engineering. Quantitative improvements of 2.6 to 500-fold have been achieved across various target compounds and host organisms through the implementation of optimized DBTL workflows. Key success factors include the integration of automated high-throughput systems, advanced computational tools for design and learning, and strategic pathway optimization based on mechanistic insights. As DBTL methodologies continue to evolve with advancements in automation, machine learning, and multi-omics technologies, further acceleration of microbial cell factory development is anticipated, enabling more sustainable and efficient bioproduction processes for a wide range of valuable chemicals.
This whitepaper details a metabolic engineering success story in which the application of a knowledge-driven Design-Build-Test-Learn (DBTL) cycle enabled the development of an Escherichia coli strain capable of producing 69.03 ± 1.2 mg/L of dopamine, a 2.6 to 6.6-fold improvement over previous state-of-the-art in vivo production methods [3]. This guide explores the principles of the DBTL cycle, the specific experimental protocols employed, and the key reagents that facilitated this advancement, providing researchers and drug development professionals with a framework for accelerating microbial strain engineering.
The Design-Build-Test-Learn (DBTL) cycle is a systematic, iterative framework central to modern synthetic biology and metabolic engineering. Its purpose is to rapidly develop and optimize microbial cell factories for the sustainable production of valuable chemicals, moving from petrochemical-dependent processes to greener, bio-based alternatives [84]. The cycle consists of four integrated phases:
- Design: In silico selection of biological parts and pathway designs.
- Build: Physical assembly of genetic constructs and engineering of microbial strains.
- Test: Cultivation of strains and high-throughput screening for target product formation.
- Learn: Data analysis to extract insights and generate hypotheses for the next cycle [5] [9].
The full automation of DBTL cycles, known as biofoundries, is becoming central to synthetic biology, yet a major challenge is the initial entry point, which often starts with limited prior knowledge [3]. The case study presented here addresses this by implementing a knowledge-driven DBTL cycle, incorporating upstream in vitro investigation to gain mechanistic understanding before embarking on extensive in vivo engineering [3].
Core Principles: The Knowledge-Driven DBTL Cycle
The knowledge-driven DBTL cycle is a rational strain engineering strategy that leverages upstream experimentation to inform the initial design phase, thereby reducing the number of iterations and resource consumption [3]. A key tool in this approach is the use of cell-free protein synthesis (CFPS) systems, particularly crude cell lysate systems. These systems bypass whole-cell constraints such as membranes and internal regulation, allowing for rapid testing of enzyme expression levels and pathway performance in a controlled environment [3]. The insights gained from these in vitro experiments are then translated into the in vivo context, enabling a more informed and efficient DBTL process.
Case Study: Optimizing Microbial Dopamine Production
Project Background and Significance
Dopamine is a valuable organic compound with applications in emergency medicine, cancer diagnosis and treatment, lithium anode production, and wastewater treatment [3]. Current industrial-scale production relies on chemical synthesis or enzymatic systems, which can be environmentally harmful and resource-intensive [3]. Developing an efficient microbial production strain offers a promising and sustainable alternative. The engineering challenge was to enhance the endogenous production of L-tyrosine in E. coli and introduce a heterologous pathway to convert it to dopamine via the intermediate L-DOPA [3].
Experimental Workflow and Pathway Engineering
The dopamine biosynthesis pathway was established in a genetically engineered E. coli host. The pathway utilizes the native metabolic network for aromatic amino acid synthesis, which was optimized to overproduce L-tyrosine. Two key enzymatic steps were introduced:
- Conversion of L-tyrosine to L-DOPA by the native E. coli enzyme 4-hydroxyphenylacetate 3-monooxygenase (HpaBC).
- Decarboxylation of L-DOPA to dopamine by a heterologous L-DOPA decarboxylase (Ddc) from Pseudomonas putida [3].
The overall experimental workflow, from initial host engineering to the final DBTL-based pathway optimization, is summarized below.
Detailed Methodologies
Host Strain Engineering for L-Tyrosine Overproduction
The base E. coli production strain (FUS4.T2) was genomically engineered to elevate the intracellular pool of L-tyrosine, the precursor for dopamine synthesis. Key modifications included [3]:
- Depletion of the TyrR regulator: The transcriptional dual regulator TyrR, which represses several genes in the aromatic amino acid biosynthesis pathway, was depleted to de-repress the pathway [3] [84].
- Mutation of feedback inhibition: The chorismate mutase/prephenate dehydrogenase (TyrA) enzyme was mutated to abolish feedback inhibition by L-tyrosine, allowing for continuous carbon flux toward the precursor [3] [3].
In Vitro Testing Using a Crude Cell Lysate System
Before in vivo DBTL cycling, the dopamine pathway was reconstituted in vitro using a crude cell lysate system [3].
- Procedure: Cell lysates were prepared from E. coli strains expressing individual pathway enzymes (HpaBC and Ddc). These lysates were combined in a reaction buffer containing essential cofactors (0.2 mM FeCl₂, 50 µM vitamin B6) and the substrate L-tyrosine (1 mM) or intermediate L-DOPA (5 mM) [3].
- Purpose: This step allowed for the independent assessment of enzyme expression and activity, helping to identify potential bottlenecks in the pathway without the complex regulatory context of a living cell [3].
In Vivo DBTL Cycle for Pathway Optimization
- Design Phase: Based on in vitro insights, a library of genetic constructs was designed to fine-tune the relative expression levels of the hpaBC and ddc genes. This was achieved through ribosome binding site (RBS) engineering, specifically by modulating the Shine-Dalgarno sequence to control the translation initiation rate (TIR) without altering secondary structures [3].
- Build Phase: The RBS library was assembled using high-throughput molecular cloning techniques, likely automated ligase cycling reaction (LCR), and transformed into the engineered E. coli production host [3] [9].
- Test Phase: The resulting library of strains was cultivated in a high-throughput 96-deepwell plate format. The cultures were grown in a defined minimal medium, induced with isopropyl β-d-1-thiogalactopyranoside (IPTG), and subsequently analyzed [3]. Quantification of dopamine and key intermediates was performed via ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) following automated extraction [3] [9].
- Learn Phase: Production data from the library was analyzed to identify the relationships between RBS sequence strength, gene expression, and final dopamine titer. This analysis revealed that the GC content in the Shine-Dalgarno sequence was a critical factor influencing RBS strength and, consequently, production efficiency [3].
Key Experimental Outcomes
The application of this knowledge-driven DBTL cycle yielded a highly efficient dopamine production strain. The quantitative results, compared to previous state-of-the-art methods, are summarized in the table below.
Table 1: Quantitative Comparison of Dopamine Production Strains
| Production Metric | State-of-the-Art (Prior to Study) | This Study (Optimized Strain) | Fold Improvement |
|---|---|---|---|
| Volumetric Titer | 27 mg/L [3] | 69.03 ± 1.2 mg/L [3] | 2.6-fold |
| Specific Yield | 5.17 mg/g~biomass~ [3] | 34.34 ± 0.59 mg/g~biomass~ [3] | 6.6-fold |
The Scientist's Toolkit: Essential Research Reagents
The successful execution of this metabolic engineering project relied on a suite of key reagents and tools. The following table details these essential components and their functions.
Table 2: Key Research Reagent Solutions for Metabolic Engineering
| Reagent / Tool | Function / Application | Specific Example from Dopamine Study |
|---|---|---|
| Microbial Chassis | Host organism for pathway engineering and chemical production. | E. coli FUS4.T2 (engineered for L-tyrosine overproduction) [3]. |
| Plasmid Vectors | Carriers for heterologous gene expression; varying copy numbers allow for tuning of gene dosage. | pET and pJNTN plasmid systems for gene expression and library construction [3]. |
| Enzymes / Genes | Code for the key catalytic steps in the biosynthetic pathway. | hpaBC (from E. coli), ddc (from Pseudomonas putida) [3]. |
| RBS Library | Fine-tunes translation initiation rate to balance metabolic flux. | A library of Shine-Dalgarno sequences to optimize expression of hpaBC and ddc [3]. |
| Cell-Free System | Crude cell lysate for rapid in vitro pathway prototyping. | Used to test enzyme expression and activity before in vivo strain construction [3]. |
| Analytical Platform | Quantifies target product and pathway intermediates with high sensitivity and speed. | UPLC-MS/MS for dopamine and L-DOPA quantification [3] [9]. |
This whitepaper has demonstrated how a knowledge-driven DBTL cycle, integrating upstream in vitro investigation with high-throughput in vivo RBS engineering, can dramatically accelerate the development of high-performance microbial cell factories. The result was a 2.6 to 6.6-fold improvement in dopamine production, showcasing the power of this rational and iterative framework.
Future efforts in this field will continue to leverage and enhance the DBTL paradigm. The integration of machine learning to analyze complex datasets from the "Learn" phase will further improve predictive design [84] [9]. The expanding toolkit for dynamic metabolic control, which allows cells to autonomously adjust flux in response to their metabolic state, presents another powerful strategy for overcoming physiological limitations and maximizing production [85]. As DBTL cycles become more automated and integrated with advanced modeling, the development of microbial cell factories for dopamine and countless other valuable chemicals will become increasingly rapid and efficient.
The Design-Build-Test-Learn (DBTL) cycle is a cornerstone methodology in synthetic biology and metabolic engineering, providing a structured framework for the development and optimization of biological systems [24]. This iterative process enables researchers to engineer microorganisms for applications ranging from drug development to the sustainable production of bio-based chemicals [37]. In metabolic engineering specifically, the DBTL cycle facilitates the systematic rewiring of microbial metabolism to enhance the production of target compounds, such as in the development of a dopamine production strain in E. coli where the DBTL approach achieved a 2.6 to 6.6-fold improvement over previous methods [37].
As biotech R&D becomes increasingly data-driven, the choice of software deployment—cloud versus on-premises—has emerged as a critical consideration for managing the vast datasets and complex workflows inherent to modern DBTL cycles [26]. This technical guide examines how these deployment models impact the efficiency, scalability, and security of DBTL management for researchers, scientists, and drug development professionals.
The DBTL Cycle: Core Components and Workflows
The DBTL cycle consists of four interconnected phases that form an iterative engineering process. The diagram below illustrates the core workflow and key outputs at each stage.
Phase-Specific Workflows and Outputs
Design Phase: Researchers plan biological systems using specialized software for protein design, genetic circuit design (including codon optimization and RBS selection), and experimental assay design [26]. This phase generates precise DNA assembly protocols specifying components such as restriction enzyme sites and assembly methods (e.g., Gibson assembly or Golden Gate cloning) [26].
Build Phase: Genetic constructs are physically assembled using molecular biology techniques such as DNA synthesis, plasmid cloning, and host organism transformation [24]. Automation integrates liquid handling robots (e.g., from Tecan, Beckman Coulter) and manages inventory systems to ensure precision and tracking [26].
Test Phase: Engineered systems undergo rigorous characterization through high-throughput screening (e.g., using plate readers like BioTek Synergy HTX), omics technologies (NGS platforms such as Illumina's NovaSeq), and biochemical assays to quantify system performance and output [24] [26].
Learn Phase: Data collected during testing is analyzed using statistical methods and machine learning algorithms to generate insights, refine hypotheses, and inform the next Design phase [26] [1]. This phase increasingly employs predictive models to forecast biological phenotypes from genotypic data [26].
Deployment Models: Technical Comparison
The effective management of DBTL cycles requires specialized software platforms, with deployment strategy significantly impacting workflow efficiency, data security, and computational scalability. The table below summarizes the key technical differences between cloud and on-premises solutions.
Table 1: Technical Comparison of Deployment Models for DBTL Management
| Aspect | Cloud Deployment | On-Premises Deployment |
|---|---|---|
| Infrastructure | Hosted on third-party servers; no physical hardware required [86] | Company-owned servers and networking equipment on-site [86] |
| Cost Structure | Subscription-based with predictable monthly fees; pay-as-you-go pricing [86] [87] | High upfront investment; potentially lower long-term costs [86] |
| Maintenance | Managed by provider (updates, patches, backups) [86] | Handled by internal IT teams, requiring expertise and resources [86] |
| Data Control | Data stored and managed by third-party provider [86] | Full control over data, with storage on local servers [86] |
| Security | Provider implements security with shared responsibility model [87] | Custom security measures tailored to business needs [86] |
| Scalability | Highly scalable; resources adjusted quickly and easily [86] | Limited scalability; requires additional hardware and time for expansion [86] |
| Accessibility | Accessible from anywhere with internet connection [88] | Limited to physical location or secured network [86] |
| Customization | Limited customization depending on provider's platform [86] | High customization potential to meet specific needs [86] |
| Compliance | Provider must meet regulatory standards; businesses have less oversight [86] | Easier to maintain compliance with industry-specific regulations [86] |
| Setup Time | Quick setup; services ready to deploy once subscribed [86] | Time-intensive setup, including hardware installation and configuration [86] |
Quantitative Impact Analysis
Research organizations can expect significantly different operational and financial outcomes based on their deployment choice:
Cost Considerations: Organizations that deploy cloud computing services save more than 35% on operating costs each year according to the Global Cloud Services Market report [89]. However, long-term subscription costs for cloud-based software can accumulate and may eventually exceed the cost of upfront software licensing fees for on-premises solutions [87].
Reliability and Uptime: Cloud providers typically guarantee at least 99.99% uptime, though occasional service interruptions can cause major problems for research workflows [87]. Sixty-one percent of SMBs experienced fewer instances of downtime and decreased length of the downtime that did occur after moving to the cloud [89].
Security Posture: Organizations that store data on-premises see 51% more security incidents than those using cloud storage, though cloud environments require proper configuration to maintain security [89].
DBTL Workflow Implementation by Deployment Model
The following diagram illustrates how deployment choices influence the practical execution of DBTL cycles, highlighting key differences in data flow and resource management.
Implementation Considerations by Deployment Type
Cloud Deployment Characteristics
Collaborative Design: Multiple researchers can concurrently access and modify genetic designs through web-based interfaces, enabling real-time collaboration across geographically dispersed teams [26] [88].
Integrated Build Phase: Cloud platforms connect directly with DNA synthesis providers (e.g., Twist Bioscience, IDT) and automate protocol generation for liquid handling systems, streamlining the transition from design to physical implementation [26].
Centralized Data Management: All experimental results from high-throughput screening and 'omics platforms are aggregated in centralized cloud repositories, facilitating standardized analysis and machine learning applications [26].
On-Premises Deployment Characteristics
Localized Design Environment: Genetic design and simulation occur on internal servers, maintaining complete data isolation and ensuring proprietary genetic constructs remain within institutional firewalls [86].
Manual Process Integration: Build and test phases rely on local inventory management and internal IT infrastructure, with data transfer between systems requiring manual intervention or custom scripting [86].
Internal Analytics: Data analysis utilizes institutional computing resources and proprietary algorithms, with no external dependency for internet connectivity or third-party software services [86] [87].
Experimental Protocols and Research Reagent Solutions
Case Study: Knowledge-Driven DBTL for Dopamine Production
Recent research demonstrates the application of a knowledge-driven DBTL cycle for developing an optimized dopamine production strain in E. coli [37]. The experimental methodology included:
In Vitro Pathway Validation: Initial testing of enzyme expression levels and dopamine pathway efficiency using crude cell lysate systems to bypass whole-cell constraints, enabling rapid iteration before in vivo implementation [37].
RBS Library Construction: Automated design and assembly of ribosomal binding site variants to fine-tune translation initiation rates for genes hpaBC (encoding 4-hydroxyphenylacetate 3-monooxygenase) and ddc (encoding L-DOPA decarboxylase) [37].
High-Throughput Screening: Cultivation of variant strains in 96-well format using minimal medium with 20 g/L glucose, followed by dopamine quantification via HPLC to identify optimal RBS combinations [37].
Machine Learning Optimization: Application of gradient boosting and random forest models to predict strain performance based on sequence features, enabling prioritization of constructs for subsequent DBTL cycles [1].
Essential Research Reagent Solutions
Table 2: Key Research Reagents and Platforms for DBTL Implementation
| Reagent/Platform | Function in DBTL Cycle | Application Example |
|---|---|---|
| Twist Bioscience DNA Synthesis | Provides custom DNA fragments for genetic construct assembly | Rapid synthesis of codon-optimized gene variants for pathway engineering [26] |
| Amicon Ultra Filters (100k MWCO) | Isolation of bacterial exosomes and extracellular vesicles | Concentration of microbial extracellular vesicles for functional studies [24] |
| Illumina NovaSeq Series | Next-generation sequencing for genotypic analysis | Comprehensive variant analysis after genome engineering or directed evolution [26] |
| BioTek Synergy HTX Multi-Mode Reader | High-throughput phenotypic screening | Quantification of fluorescent protein expression or metabolic output in 384-well format [26] |
| TeselaGen LIMS Platform | End-to-end DBTL cycle management | Orchestration of design, build, test, and learn phases with automated data integration [26] |
| CRISPR-Cas9 Genome Editing | Precision genetic modifications in host strains | Knockout of competitive pathways or regulatory elements in production hosts [37] |
| Cell-Free Protein Synthesis Systems | In vitro prototyping of metabolic pathways | Rapid testing of enzyme combinations without cellular constraints [37] |
The choice between cloud and on-premises deployment for DBTL management represents a significant strategic decision with far-reaching implications for research efficiency, data security, and innovation velocity in metabolic engineering. Cloud solutions offer unparalleled collaboration capabilities, dynamic scalability, and reduced IT overhead, making them particularly suitable for multi-institutional collaborations and rapidly evolving research programs. Conversely, on-premises deployments provide maximum data control, regulatory compliance simplicity, and potentially lower long-term costs for stable, well-defined research workflows with sensitive intellectual property considerations.
As DBTL cycles become increasingly automated through biofoundries and integrated AI platforms [27], the optimal deployment strategy may evolve toward hybrid approaches that leverage the strengths of both models. Ultimately, the selection between cloud and on-premises solutions should be guided by specific research requirements, regulatory constraints, and organizational capabilities, with the understanding that this infrastructure decision will fundamentally shape the efficiency and effectiveness of metabolic engineering research programs.
The design-build-test-learn (DBTL) cycle is a foundational framework in metabolic engineering for the iterative development of microbial cell factories. Each revolution of the cycle aims to bring scientists closer to an optimal strain for producing a target compound, such as a therapeutic drug or bio-based chemical. However, traditional DBTL cycles are often hampered by their slow pace, high resource consumption, and reliance on intuitive, experience-based decisions. The integration of automation and machine learning (ML) is fundamentally transforming this process, introducing unprecedented levels of efficiency and data-driven insight. This technical guide examines the quantitative benefits and detailed methodologies of applying automation and ML within the DBTL cycle, providing researchers and drug development professionals with a roadmap for implementation. By leveraging these technologies, laboratories can accelerate the development of critical bioprocesses, from novel drug candidates to sustainable production platforms.
The DBTL Cycle: A Framework for Accelerated Strain Engineering
The DBTL cycle provides a structured, iterative approach to strain optimization. Its four phases form a closed loop that systematically incorporates learning from one iteration to inform the design of the next.
- Design: In this initial phase, scientists plan genetic modifications. This can involve selecting enzymes, promoters, ribosomal binding sites (RBS), and other genetic parts to create a library of potential strain designs. The challenge is navigating a vast combinatorial space; for example, a pathway with 5 enzymes, each with 5 possible expression levels, creates 3,125 (5⁵) potential variants. Testing all possibilities is experimentally infeasible.
- Build: This phase involves the physical construction of the designed genetic variants within the host organism (e.g., E. coli or yeast). Automated biofoundries are crucial here, using techniques like high-throughput molecular cloning and genome editing to assemble dozens to hundreds of strains in parallel.
- Test: The constructed strains are cultured, and their performance is characterized. Key performance indicators (KPIs) such as titer, yield, and productivity (TYR) are measured. Automation enables high-throughput analytics, including liquid handling robots for culturing and chromatography systems for metabolite quantification.
- Learn: Data from the test phase are analyzed to extract meaningful insights. This is where machine learning becomes powerful. ML models learn the complex relationships between genetic designs (inputs) and strain performance (outputs), identifying non-intuitive optima that escape rational design.
A key challenge in traditional DBTL cycles is the combinatorial explosion of possible designs. ML helps navigate this space intelligently. As one study notes, "combinatorial pathway optimization is therefore often performed using iterative DBTL cycles. The aim of these cycles is to develop a product strain iteratively, every time incorporating learning from the previous cycle" [1].
Quantifying the Impact: Automation and ML in the DBTL Cycle
The integration of automation and ML introduces significant efficiencies across the DBTL cycle. The following tables summarize the quantitative and qualitative impacts on key metrics and cycle components.
Table 1: Quantitative Benefits of Automation and ML in Metabolic Engineering
| Metric | Traditional Approach | With Automation & ML | Improvement | Source/Case Study |
|---|---|---|---|---|
| Strain Development Time | Manual cloning and screening | Automated biofoundries & ML-guided design | Cycle time reduced by weeks to months | [3] [71] |
| Data Scientist Time on Data Prep | ~39% of time spent on data preparation | AutoML automates feature engineering and preprocessing | Significant reduction in manual labor | [90] |
| Model Development Speed | Manual model selection and tuning | Automated Machine Learning (AutoML) | Development timeline accelerated 6x (PayPal case) | [90] |
| Production Titer | Baseline (e.g., 27 mg/L dopamine) | Knowledge-driven DBTL with high-throughput RBS engineering | 2.6 to 6.6-fold increase (69 mg/L dopamine) | [3] |
| Pathway Optimization | Sequential, intuitive debottlenecking | Combinatorial optimization guided by ML models | Identifies non-intuitive global optima | [1] |
Table 2: Impact of Automation and ML on Individual DBTL Phases
| DBTL Phase | Impact of Automation | Impact of Machine Learning |
|---|---|---|
| Design | Automated design software using standards like SBOL. | ML models recommend high-performing designs, balancing exploration/exploitation. |
| Build | Robotic liquid handlers, automated DNA assembly, and strain construction. | Not directly applicable, but ML can optimize build protocols. |
| Test | High-throughput culturing (e.g., microbioreactors) and automated analytics (HPLC, MS). | ML improves experimental design (e.g., selecting informative strains to test). |
| Learn | Automated data pipelines and databases. | ML (e.g., gradient boosting) extracts insights from high-dimensional data, generating testable hypotheses. |
The application of a knowledge-driven DBTL cycle for dopamine production in E. coli exemplifies these benefits. By combining upstream in vitro tests with high-throughput RBS engineering, researchers developed a strain producing 69.03 ± 1.2 mg/L of dopamine, a 2.6 and 6.6-fold improvement over previous state-of-the-art in vivo production methods [3]. This demonstrates how a structured, automated approach can dramatically enhance outcomes.
Detailed Experimental Protocols for an Automated ML-Driven DBTL Cycle
This section outlines a generalized protocol for implementing an automated, ML-guided DBTL cycle, based on successful case studies in the literature.
Protocol 1: Initial Library Design and High-Throughput Screening
Objective: To build and test an initial diverse library of strain variants for generating a foundational dataset for ML model training.
- Pathway Identification: Identify the target metabolic pathway (e.g., dopamine synthesis from L-tyrosine [3]).
- Genetic Part Selection: Create a library of genetic parts (promoters, RBS sequences, gene homologs) known to affect enzyme expression and activity.
- Automated Library Design: Use computational tools (e.g., UTR Designer [3]) to generate a diverse set of genetic constructs. To avoid bias, design a library that samples the expression space widely rather than clustering around expected optima.
- High-Throughput Build Phase:
- High-Throughput Test Phase:
- Employ liquid handling robots to inoculate and cultivate variants in deep-well plates or microbioreactors.
- Monitor growth (OD600) and product formation online or via end-point assays.
- Use automated analytical platforms (e.g., HPLC, FIA, LC-MS/MS [71]) to quantify key metabolites (substrate, product, by-products).
Protocol 2: Machine Learning Model Training and Design Recommendation
Objective: To learn from the initial screening data and recommend a new, improved set of strains for the next DBTL cycle.
- Data Preprocessing and Feature Engineering:
- Input Features (X): Encode each genetic design numerically. This can include one-hot encoding for categorical choices (e.g., promoter type) and continuous values for expression strengths (e.g., predicted TIR from RBS sequences) [1] [3].
- Output/Target (Y): Use the measured KPIs from the Test phase (e.g., product titer, yield, biomass) [1].
- Clean the data by handling missing values and normalizing features.
- Model Training and Selection:
- Train multiple ML models, including Gradient Boosting, Random Forest, and Support Vector Machines (SVMs). Studies show that Gradient Boosting and Random Forest often outperform other methods in the low-data regime typical of early DBTL cycles [1].
- Use a hold-out test set or cross-validation to evaluate model performance (e.g., using R² score or Mean Absolute Error).
- Select the best-performing model for generating recommendations.
- Recommendation Algorithm:
- Use the trained model to predict the performance of a vast number of in silico strain designs.
- Implement an acquisition function (e.g., Expected Improvement) to select the next set of strains to build. This function balances exploitation (choosing designs predicted to be high-performing) and exploration (choosing designs where the model is uncertain) [1].
- Output a list of top candidate genetic designs for the next Build phase.
Case Study: Dopamine Production in E. coli
The "knowledge-driven DBTL" cycle for dopamine production provides a concrete example of these protocols in action [3].
- Host Strain Engineering: The production host (E. coli FUS4.T2) was first engineered for high L-tyrosine production by deleting the transcriptional regulator tyrR and mutating the feedback inhibition of tyrA [3].
- In Vitro Precursor Investigation: Before the first in vivo DBTL cycle, the pathway enzymes (HpaBC and Ddc) were tested in a crude cell lysate CFPS system. This in vitro step provided rapid feedback on enzyme activity and interactions, de-risking the initial in vivo design [3].
- In Vivo Fine-Tuning: The relative expression levels of hpaBC and ddc were optimized in vivo by constructing a library of bicistronic constructs with varying RBS strengths. This high-throughput RBS engineering allowed for precise tuning of the metabolic flux [3].
- Analytical Methods: Dopamine and L-tyrosine concentrations were quantified using HPLC, demonstrating the use of automated analytical platforms for high-fidelity testing [3].
Visualizing the Workflow and Pathway
The following diagrams, generated with Graphviz, illustrate the logical workflow of an integrated DBTL cycle and a specific metabolic pathway optimized using this approach.
Automated ML-Driven DBTL Cycle
Dopamine Biosynthesis Pathway
The Scientist's Toolkit: Essential Research Reagents and Solutions
Successful implementation of an automated, ML-driven DBTL cycle relies on a suite of specialized reagents, tools, and platforms.
Table 3: Key Research Reagent Solutions for an Automated DBTL Cycle
| Item | Function | Example/Description |
|---|---|---|
| RBS Library | Fine-tunes translation initiation rate and relative enzyme expression levels in a pathway. | A set of sequences modulating the Shine-Dalgarno sequence; crucial for balancing flux in pathways like dopamine synthesis [3]. |
| Promoter Library | Provides varying levels of transcriptional control for genes of interest. | A collection of constitutive or inducible promoters (e.g., based on Ptac) with different strengths [1]. |
| Engineered Host Strain | Provides a high-flux background for the heterologous pathway, often with precursor overproduction. | e.g., E. coli FUS4.T2 with tyrR deletion and feedback-inhibition-resistant tyrA for L-tyrosine overproduction [3]. |
| Automated Liquid Handling System | Executes repetitive pipetting tasks with high precision and speed for the Build and Test phases. | Platforms from Hamilton, Tecan, or Beckman Coulter for cloning, transformation, and culturing. |
| Cell-Free Protein Synthesis (CFPS) System | Enables rapid in vitro testing of enzyme combinations and pathway logic before in vivo implementation. | Crude E. coli cell lysate containing transcription/translation machinery [3]. |
| AutoML Platform | Automates the end-to-end process of building and selecting high-performing ML models. | Platforms like H2O.ai, Google Cloud AutoML, or Auto-SKLearn [90]. |
| Kinetic Model | A mechanistic model used in silico to simulate pathway behavior and benchmark ML methods. | e.g., A model built with the SKiMpy package, integrating a synthetic pathway into an E. coli core kinetic model [1]. |
The integration of automation and machine learning within the DBTL cycle marks a paradigm shift in metabolic engineering and drug development. This guide has detailed how this synergy delivers quantifiable reductions in development time and resource consumption while simultaneously enhancing final product titers and yields. The transition from a manual, intuition-driven process to an automated, data-driven one allows researchers to efficiently navigate vast combinatorial spaces, uncovering non-intuitive optimal solutions. As these technologies continue to mature—with advances in AutoML, more sophisticated robotic biofoundries, and improved data integration—their impact will only grow. For research organizations aiming to accelerate the development of novel therapeutics and sustainable bioprocesses, the strategic adoption of automated, ML-powered DBTL cycles is no longer a futuristic concept but a present-day imperative for maintaining a competitive edge.
Conclusion
The DBTL cycle represents a paradigm shift in metabolic engineering, moving from sequential, intuition-based approaches to a systematic, data-driven, and iterative framework. The key takeaways underscore that successful implementation hinges on the tight integration of all four phases, powered by automation, sophisticated data management, and advanced machine learning. As demonstrated by numerous case studies, this methodology consistently leads to significant performance enhancements, achieving multi-fold increases in product titers. The future of DBTL points towards increasingly autonomous biofoundries, where AI not only recommends designs but also manages the entire cycle. For biomedical and clinical research, these advancements promise to drastically accelerate the development of novel microbial cell factories for the sustainable production of vital drugs, therapeutic molecules, and diagnostic agents, ultimately reshaping the landscape of biomanufacturing and therapeutic discovery.
References
- 1. Simulated Design–Build–Test–Learn Cycles for Consistent ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10510747/]
- 2. A generalized platform for artificial intelligence-powered ... [https://www.nature.com/articles/s41467-025-61209-y]
- 3. The knowledge driven DBTL cycle provides mechanistic ... [https://microbialcellfactories.biomedcentral.com/articles/10.1186/s12934-025-02729-6]
- 4. RespectM revealed metabolic heterogeneity powers deep ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10329182/]
- 5. Recent advances in the Design-Build-Test-Learn (DBTL ... [https://pubmed.ncbi.nlm.nih.gov/40195836/]
- 6. Engineering/Learn - 2021.igem.org [https://2021.igem.org/Engineering/Learn]
- 7. Iterative DBTL Cycles - 2025 UBC iGEM Wiki [https://2025.igem.wiki/ubc-vancouver/inclusive-design-project/iterative-dbtl-cycles]
- 8. Combinatorial pathway optimization for streamlined ... [https://www.sciencedirect.com/science/article/abs/pii/S0958166917300472]
- 9. An automated Design-Build-Test-Learn pipeline for ... [https://www.nature.com/articles/s42003-018-0076-9]
- 10. Multiomics Data Collection, Visualization, and Utilization ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7902046/]
- 11. The Design-Build-Test-Learn cycle for metabolic of... engineering [https://orbit.dtu.dk/en/publications/the-design-build-test-learn-cycle-for-metabolic-engineering-of-st]
- 12. DBTL Approach in Synthetic Biology [https://www.moleculardevices.com/applications/synthetic-biology/dbtl-approach]
- 13. The knowledge driven DBTL provides mechanistic insights while... cycle [https://link.springer.com/article/10.1186/s12934-025-02729-6]
- 14. Simulated Design-Build-Test-Learn Cycles for Consistent Comparison... [https://pubmed.ncbi.nlm.nih.gov/37616156/]
- 15. Visualizing metabolic network dynamics through time-series ... [https://bmcbioinformatics.biomedcentral.com/articles/10.1186/s12859-020-3415-z]
- 16. Effective data visualization strategies in untargeted ... [https://pubs.rsc.org/en/content/articlehtml/2025/np/d4np00039k]
- 17. Escherichia coli as a host for metabolic engineering [https://www.sciencedirect.com/science/article/abs/pii/S1096717618300740]
- 18. Lessons from Two Design-Build-Test-Learn Cycles of ... [https://pubmed.ncbi.nlm.nih.gov/31072100/]
- 19. System metabolic engineering of Escherichia coli W for the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10158823/]
- 20. Tools for metabolic engineering in Streptomyces - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC4156490/]
- 21. Streptomyces as Microbial Chassis for Heterologous ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8724576/]
- 22. Development and validation of an updated computational ... [https://bmcgenomics.biomedcentral.com/articles/10.1186/s12864-018-4905-5]
- 23. ActinoMation: A literate programming approach for medium ... [https://www.sciencedirect.com/science/article/pii/S2405805X25000389]
- 24. Engineering | ESSENTIAL KOREA - iGEM 2025 [https://2025.igem.wiki/essential-korea/engineering]
- 25. methods in Computational for metabolic engineering strain design [https://pubmed.ncbi.nlm.nih.gov/25576846/]
- 26. DBTL Cycle Advancement: Software Elevates Biotech R&D [https://teselagen.com/blog/revolutionizing-rd-dbtl-cycle/]
- 27. Abstraction hierarchy to define biofoundry workflows and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12214803/]
- 28. A DNA toolkit to unlock the CRISPR/Cas9 potential for... assembly [https://www.nature.com/articles/s42003-023-05202-5?error=cookies_not_supported&code=a1b032f2-4822-4be1-a7a6-ddea9c2a2906]
- 29. Engineering - Iterative DBTL Cycles | LYON - iGEM 2025 [https://2025.igem.wiki/lyon/engineering]
- 30. Machine learning-led semi-automated medium ... [https://www.nature.com/articles/s42003-025-08039-2]
- 31. CRISPR/Cas9 Systems for the Development of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7710542/]
- 32. Recent advances in high-throughput metabolic engineering [https://www.sciencedirect.com/science/article/abs/pii/S0734975022000660]
- 33. High-throughput functional genomics using CRISPR-Cas9 [https://pmc.ncbi.nlm.nih.gov/articles/PMC4503232/]
- 34. Multi-functional genome-wide CRISPR system for high ... [https://www.nature.com/articles/s41467-019-13621-4]
- 35. How Phenotypic CRISPR Screening is Revolutionising ... [https://www.ddw-online.com/how-phenotypic-crispr-screening-is-revolutionising-drug-discovery-1424-201810/]
- 36. Technologies for design-build-test-learn automation and ... [https://link.springer.com/article/10.1007/s13721-024-00455-4]
- 37. The knowledge driven DBTL cycle provides mechanistic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12084978/]
- 38. A Comprehensive Review of High Throughput ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9590503/]
- 39. Automated phenotyping of microalgae: scalable solution ... [https://www.sciencedirect.com/science/article/pii/S0960852425007291]
- 40. Metabolic Phenotyping - HSC Cores - The University of Utah [https://cores.utah.edu/metabolic-phenotyping/]
- 41. Breaking through the bottleneck in future crop breeding [https://www.sciencedirect.com/science/article/pii/S2214514121000829]
- 42. Questions, data and models underpinning metabolic ... [https://www.frontiersin.org/journals/systems-biology/articles/10.3389/fsysb.2022.998048/full]
- 43. Modeling for understanding and engineering metabolism - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC11894412/]
- 44. Model-guided dynamic control of essential metabolic ... [https://www.sciencedirect.com/science/article/pii/S1096717621001245]
- 45. A review on design-build-test-learn cycle to potentiate ... [https://www.sciencedirect.com/science/article/abs/pii/S0960852422013141]
- 46. The Design-Build-Test-Learn cycle for metabolic ... [https://pubmed.ncbi.nlm.nih.gov/33956071/]
- 47. ISOPRENOID PATHWAY OPTIMIZATION FOR TAXOL ... - NCBI [https://www.ncbi.nlm.nih.gov/books/NBK84464/]
- 48. Metabolic engineering for the high-yield production of ... [https://www.nature.com/articles/srep11128]
- 49. Enhancing isoprenol production by systematically tuning ... [https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2023.1296132/full]
- 50. Exploring metabolic engineering design principles for the ... [https://biotechnologyforbiofuels.biomedcentral.com/articles/10.1186/1754-6834-7-99]
- 51. Enhanced isoprenoid production in Escherichia coli cells ... [https://www.sciencedirect.com/science/article/pii/S2405580825003942]
- 52. 综述:生物铸造厂前沿:机遇与挑战 [https://www.ebiotrade.com/newsf/2025-10/20251006103932830.htm]
- 53. 合成生物学核心功能及技术原理介绍 [https://www.synceres.com/news_details/14.html]
- 54. 综述:由人工智能驱动的生物铸造厂,用于蛋白质工程和代谢 ... [https://www.ebiotrade.com/newsf/2025-11/20251107084831363.htm]
- 55. 天津工业生物技术研究所成功开发新型代谢工程靶点设计算法 [https://bydrug.pharmcube.com/news/detail/3a23297a25e9c2d84e3df0d0c685d0ca]
- 56. 機器學習與無細胞測試革新生物工程設計 [https://geneonline.news/%E6%A9%9F%E5%99%A8%E5%AD%B8%E7%BF%92%E8%88%87%E7%84%A1%E7%B4%B0%E8%83%9E%E6%B8%AC%E8%A9%A6%E9%9D%A9%E6%96%B0%E7%94%9F%E7%89%A9%E5%B7%A5%E7%A8%8B%E8%A8%AD%E8%A8%88/]
- 57. Engineering biological systems using automated ... [https://www.sciencedirect.com/science/article/abs/pii/S1096717617300502]
- 58. Pareto optimal metabolic engineering for the growth‐coupled ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9321710/]
- 59. RespectM revealed metabolic heterogeneity powers deep ... [https://pubmed.ncbi.nlm.nih.gov/37426353/]
- 60. LDBT instead of DBTL: combining machine learning and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12589603/]
- 61. Recent advances in machine learning applications ... [https://www.sciencedirect.com/science/article/abs/pii/S0734975022001653]
- 62. A machine learning approach to predict metabolic pathway ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5974308/]
- 63. SCOUR: a stepwise machine learning framework for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8268592/]
- 64. Machine learning to predict metabolic drug interactions ... [https://jcheminf.biomedcentral.com/articles/10.1186/s13321-022-00602-x]
- 65. Learning the way toward high-precision biological design [https://pmc.ncbi.nlm.nih.gov/articles/PMC10231942/]
- 66. Comparison of Random Forest and Gradient Boosting ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8392226/]
- 67. Recent advances in systems metabolic engineering [https://www.sciencedirect.com/science/article/abs/pii/S0958166923001143]
- 68. Comparative Study of Random Forest and Gradient ... [https://www.mdpi.com/2673-4591/59/1/24]
- 69. A Computational Framework to Identify Metabolic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8777033/]
- 70. DBTL Cycles | WIST - iGEM 2025 [https://2025.igem.wiki/wist/dbtlcycles]
- 71. Automating the design-build-test-learn cycle towards next- ... [https://www.sciencedirect.com/science/article/pii/S187167842300002X]
- 72. Metabolic pathway + enzyme engineering [https://telesisbio.com/applications/synthetic-dna/metabolic-pathway-enzyme-engineering/]
- 73. Machine learning for metabolic engineering: A review [https://www.sciencedirect.com/science/article/pii/S109671762030166X]
- 74. combining machine learning and rapid cell-free testing [https://www.nature.com/articles/s41467-025-65281-2]
- 75. DNA synthesis technologies to close the gene writing gap - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC9869848/]
- 76. Enzymatic DNA synthesis: Moving beyond limits [https://www.ddw-online.com/enzymatic-dna-synthesis-moving-beyond-limits-36071-202508/]
- 77. Quantification of synthetic errors during chemical synthesis ... [https://www.nature.com/articles/s41598-022-16222-2]
- 78. Overcoming DNA Synthesis Challenges in Next-Gen ... [https://www.genengnews.com/sponsored/overcoming-dna-synthesis-challenges-in-next-gen-genetic-medicines/]
- 79. Synthetic biology and metabolic engineering paving the way ... [https://pubs.rsc.org/en/content/articlehtml/2025/ya/d5ya00118h]
- 80. Simulated Design-Build-Test-Learn cycles [https://www.ai4b.io/news-and-events/news-2/a-testnew-blog-post/]
- 81. AbeelLab/simulated-dbtl [https://github.com/AbeelLab/simulated-dbtl]
- 82. Design-Build-Test-Learn Workflows - pySBOL's documentation! [https://pysbol2.readthedocs.io/en/latest/dbtl.html]
- 83. Improving metabolic engineering design with enzyme ... [https://www.sciencedirect.com/science/article/pii/S1096717625000850]
- 84. Metabolic engineering: Tools and applications - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC11610952/]
- 85. Dynamic control in metabolic engineering: Theories, tools ... [https://www.sciencedirect.com/science/article/pii/S1096717620301440]
- 86. On-Premises (On-Prem): Benefits, Limitations, and More [https://www.splashtop.com/blog/on-premises]
- 87. On premises vs. cloud pros and cons, key differences [https://www.techtarget.com/searchcloudcomputing/tip/Evaluate-on-premises-vs-cloud-computing-pros-and-cons]
- 88. The Advantages and Disadvantages of Cloud Computing [https://info.cybertecsecurity.com/advantages-and-disadvantages-of-cloud-computing]
- 89. What are the Pros & Cons of Cloud Computing? - Morefield [https://morefield.com/blog/pros-and-cons-of-cloud-computing/]
- 90. Benefits of Automated Machine Learning: The Full Guide [https://indatalabs.com/blog/automated-machine-learning]